Abstract
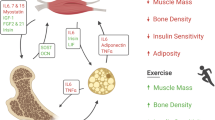
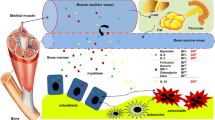
Several lines of evidence have recently established that skeletal muscle is an endocrine organ producing and releasing myokines acting in a paracrine or endocrine fashion. Among these, the newly identified myokine Irisin, produced by skeletal muscle after physical exercise, was originally described as molecule able to promote energy expenditure in white adipose tissue. Recently, it has been shown that the myokine Irisin affects skeletal metabolism in vivo. Thus, mice treated with a micro-dose of r-Irisin displayed improved cortical bone mass, geometry and strength, resembling the effect of physical activity in developing an efficient load-bearing skeleton. Further studies highlighted the autocrine effect of Irisin on skeletal muscle, and research performed in humans has definitively established that Irisin is a circulating hormone-like myokine, increased by physical activity. Albeit there are still few, since Irisin has been very recently discovered, herein are summarized the most relevant research findings published on this topic.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yang J. Enhanced skeletal muscle for effective glucose homeostasis. Progress in molecular biology and translational science. Academic Press. 2014; p. 133–63.
Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–82.
DiGirolamo DJ, Kiel DP, Esser KA. Bone and skeletal muscle: neighbors with closeties. J Bone Miner Res. 2013;28:1509–18.
Karsenty G, Oury F. Biology without walls: the novel endocrinology of bone. Annu Rev Physiol. 2012;74:87–105.
Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle–bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–5.
Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007;1116:281–90.
Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–15.
Liu R, Schindeler A, Little DG. The potential role of muscle in bone repair. J Musculoskelet Neuronal Interact. 2010;10(1):71–6.
Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65.
Colaianni G, Cuscito C, Mongelli T, Oranger A, Mori G, Brunetti G, et al. Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol. 2014;2014, 902186. This is the first study showing that the myokine Irisin, produced by skeletal muscle, acts directly on osteoblasts.
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The Myokine Irisin increases cortical bone mass. PNAS. 2015;112(39):12157–62. This study demonstrated that the treatment with low-dose of recombinant Irisin improves cortical mineral density, geometry and strength in bone of young healthy mice.
Colaianni G, Grano M. Role of Irisin on the bone-muscle functional unit. Bonekey Rep. 2015;4:765.
Holmes D. Bone: Irisin boosts bone mass. Nat Rev Endocrinol. 2015;11(12):689.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Zhang Y, Li R, Meng Y, Li S, Donelan W, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63(2):514–25. This study showed that the treatment with high dose of recombinant Irisin induces the browning expansion in white adipose tissue, reducing body weight and improving glucose homeostasis.
Baxter-Jones AD, Kontulainen SA, Faulkner RA, Bailey DA. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone. 2008;43(6):1101–7.
Toma CD, Ashkar S, Gray ML, Schaffer JL, Gerstenfeld LC. Signal transduction of mechanical stimuli is dependent on microfilament integrity: identification of osteopontin as a mechanically induced gene in osteoblasts. J Bone Miner Res. 1997;12(10):1626–36.
Harter LV, Hruska KA, Duncan RL. Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology. 1995;136(2):528–35.
Kubota T, Yamauchi M, Onozaki J, Sato S, Suzuki Y, Sodek J. Influence of an intermittent compressive force on matrix protein expression by ROS 17/2.8 cells, with selective stimulation of osteopontin. Arch Oral Biol. 1993;38(1):23–30.
Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2013;25(9):1897–904.
Lin C, Jiang X, Dai Z, Guo X, Weng T, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24(10):1651–61.
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–9.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–75.
Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin W, et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep. 2016;6:21053.
Singhal V, Lawson EA, Ackerman KE, Fazeli PK, Clarke H, et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLoS One. 2014;9(6), e100218.
Palermo A, Strollo R, Maddaloni E, Tuccinardi D, D’Onofrio L, et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin Endocrinol (Oxf). 2015;82(4):615–9.
Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, et al. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int. 2014;25(5):1633–42.
Klangjareonchai T, Nimitphong H, Saetung S, Bhirommuang N, Samittarucksa R, et al. Circulating sclerostin and irisin are related and interact with gender to influence adiposity in adults with prediabetes. Int J Endocrinol. 2014;2014, 261545.
Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, et al. Irisin - a myth rather than an exercise-inducible myokine. Sci Rep. 2015;5:8889.
Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015. doi:10.1016/j.cmet.2015.08.001. This work provides evidence that human Irisin is detectable in the circulation and its synthesis is upregulated by physical activity.
Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8(4), e60563.
Vaughan RA, Gannon NP, Mermier CM, Conn CA. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J Physiol Biochem. 2015;71(4):679–89.
Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond). 2014;38(12):1538–44.
Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013;27(5):1981–9. This work showed that the myokines Irisin and myostatin are inversely regulated in skeletal muscle.
Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010;10(1):56–63.
MacKenzie MG, Hamilton DL, Pepin M, Patton A, Baar K. Inhibition of myostatin signaling through Notch activation following acute resistance exercise. PLoS One. 2013;8(7), e68743.
Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 2013;154(8):2687–701. This work demonstrated that transgenic mice overexpressing FoxC2 in adipose tissues, a murine model for BAT induction, displayed high bone mass due to increased bone formation, triggered by wingless related MMTV integration site 10b (WNT10b) and insulin-like growth factor binding protein 2 (IGFBP2), released from BAT.
Graja A, Schulz TJ. Mechanisms of aging-related impairment of brown adipocyte development and function. Gerontology. 2015;61(3):211–7.
Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23(9):3113–20.
Acknowledgments
This work was supported in part by an ERISTO grant (to M.G.), a MIUR grant ex60% (to M.G.) and a SIOMMMS grant (to G.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
M. Grano, S. Cinti, Dr. Mongelli, S. Colucci and G. Colainni report grants from ERISTO, grants from MIUR ex60%, and grants from SIOMMMS, during the conduct of the study. In addition, S. Cinti, S. Colucci and G. Colainni have a patent, MI2015A000558.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Additional information
This article is part of the Topical Collection on Muscle and Bone
Rights and permissions
About this article
Cite this article
Colaianni, G., Mongelli, T., Colucci, S. et al. Crosstalk Between Muscle and Bone Via the Muscle-Myokine Irisin. Curr Osteoporos Rep 14, 132–137 (2016). https://doi.org/10.1007/s11914-016-0313-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-016-0313-4