Abstract
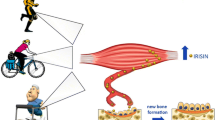
Irisin is a myokine secreted by the skeletal muscle during physical activity both in mice and humans. Its first identified role was to activate the browning response in white adipocytes, subsequently triggering non-shivering thermogenesis; therefore, Irisin has raised great expectations as a potential target in the treatment of obesity. In 2015, we demonstrated that Irisin plays a central role in the control of bone mass, driving positive effects on cortical mineral density and bone mechanical properties. This effect on the bone was triggered using an Irisin dosage 70 times lower than the one needed to induce the browning response, suggesting that the skeleton is the primary target organ of this myokine. Moreover, our studies also highlighted the autocrine effect of Irisin on the skeletal muscle, overall suggesting that Irisin plays a fundamental role in the physiology of the musculoskeletal system. More recently, we demonstrated the efficacy of Irisin in preventing and restoring bone and muscle losses in a mouse model affected by disuse-induced osteoporosis and muscular atrophy. Hopefully, if future investigations will be confirmed in humans, it may lead to develop an Irisin-based therapy for physically disable or bedridden patients and it might also represent a countermeasure for astronauts subjected to microgravity.

Similar content being viewed by others
References
Dunstan D. Diabetes: exercise and T2DM-move muscles more often! Nat Rev Endocrinol. 2011;7(4):189–90. https://doi.org/10.1038/nrendo.2011.35.
Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17(2):162–84. https://doi.org/10.1016/j.cmet.2012.12.012.
Zehnder Y, Lüthi M, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15(3):180–9. https://doi.org/10.1007/s00198-003-1529-6.
Jørgensen L, Jacobsen BK, Wilsgaard T, Magnus JH. Walking after stroke: does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos Int. 2000;11(5):381–7. https://doi.org/10.1007/s001980070103.
Oppl B, Michitsch G, Misof B, Kudlacek S, Donis J, Klaushofer K, et al. Low bone mineral density and fragility fractures in permanent vegetative state patients. J Bone Miner Res. 2014;29(5):1096–100. https://doi.org/10.1002/jbmr.2122.
Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19(4):319–38. https://doi.org/10.1615/CritRevEukarGeneExpr.v19.i4.50.
Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway. JBMR Plus. 2017;1(2):86–100. https://doi.org/10.1002/jbm4.10015.
Keller P, Vollaard NB, Gustafsson T, Gallagher IJ, Sundberg CJ, Rankinen T, et al. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol. 2011;110(1):46–59. https://doi.org/10.1152/japplphysiol.00634.2010.
Pedersen BK, Akerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103(3):1093–8. https://doi.org/10.1152/japplphysiol.00080.2007.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. https://doi.org/10.1038/nature10777.
Colaianni G, Cuscito C, Mongelli T, Oranger A, Mori G, Brunetti G, et al. Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol. 2014;902186
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112(39):12157–62. https://doi.org/10.1073/pnas.1516622112.
Colaianni G, Grano M. Role of Irisin on the bone-muscle functional unit. Bonekey Rep. 2015;4:765.
Holmes D. Bone: Irisin boosts bone mass. Nat Rev Endocrinol. 2015;11:689.
Lieberman DE, Polk JD, Demes B. Predicting long bone loading from cross-sectional geometry. Am J Phys Anthropol. 2004;123(2):156–71. https://doi.org/10.1002/ajpa.10316.
Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204–8. https://doi.org/10.2106/00004623-197759020-00012.
Vaughan RA, Gannon NP, Mermier CM, Conn CA. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J Physiol Biochem. 2015;71(4):679–89. https://doi.org/10.1007/s13105-015-0433-9.
Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes. 2014;38:1538–44.
MacKenzie MG, DL Hamilton, M Pepin, A Patton, K Baar. Inhibition of myostatin signaling through Notch activation following acute resistance exercise. PLoS One 2013; 8(7): e68743. https://doi.org/10.1371/journal.pone.0068743.
Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010;10(1):56–63.
Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013;27(5):1981–9. https://doi.org/10.1096/fj.12-225755.
LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskeletel Neuronal Interact. 2007;7:33–47.
Karasik D, Kiel DP. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone. 2010;46(5):1226–37. https://doi.org/10.1016/j.bone.2010.01.382.
Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Muir SW, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc. 2015 Apr;16(4):290–5. https://doi.org/10.1016/j.jamda.2014.10.018.
Crepaldi G, Maggi S. Sarcopenia and osteoporosis: a hazardous duet. J Endocrinol Investig. 2005;28(10 Suppl):66–8.
LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, et al. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact. 2000;1(2):157–60.
Riggs BL, Khosla S, Melton LJ 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13(5):763–73. https://doi.org/10.1359/jbmr.1998.13.5.763.
Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol. 2005;93(4):463–8. https://doi.org/10.1007/s00421-004-1236-9.
LeBlanc A, Schneider V. Countermeasures against space flight related bone loss. Acta Astronaut. 1992;27:89–92. https://doi.org/10.1016/0094-5765(92)90182-I.
Morey-Holton E, Globus RK, Kaplansky A, Durnova G. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med. 2005;10:7–40. https://doi.org/10.1016/S1569-2574(05)10002-1.
Allen MR, Bloomfield SA. Hindlimb unloading has a greater effect on cortical compared with cancellous bone in mature female rats. J Appl Physiol. 2003;94(2):642–50. https://doi.org/10.1152/japplphysiol.00656.2002.
Swift JM, Nilsson MI, Hogan HA, Sumner LR, Bloomfield SA. Simulated resistance training during hindlimb unloading abolishes disuse bone loss and maintains muscle strength. J Bone Miner Res. 2010;25(3):564–74. https://doi.org/10.1359/jbmr.090811.
Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 2013;28(4):865–74. https://doi.org/10.1002/jbmr.1807.
Gerbaix M, Vico L, Ferrari SL, Bonnet N. Periostin expression contributes to cortical bone loss during unloading. Bone. 2015;71:94–100. https://doi.org/10.1016/j.bone.2014.10.011.
Jing D, Cai J, Wu Y, Shen G, Li F, Xu Q, et al. Pulsed electromagnetic fields partially preserve bone mass, microarchitecture, and strength by promoting bone formation in hindlimb-suspended rats. J Bone Miner Res. 2014;29(10):2250–26. https://doi.org/10.1002/jbmr.2260.
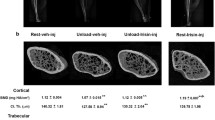
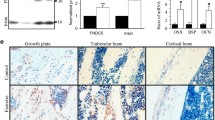
Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep. 2017;7(1):2811. https://doi.org/10.1038/s41598-017-02557-8.
Alberich-Bayarri A, Marti-Bonmati L, Pérez MA, Sanz-Requena R, Lerma-Garrido JJ, García-Martí G, et al. Assessment of 2D and 3D fractal dimension measurements of trabecular bone from high-spatial resolution magnetic resonance images at 3 T. Med Phys. 2010;37(9):4930–7. https://doi.org/10.1118/1.3481509.
Majumdar S, Genant HK, Grampp S, Newitt DC, Truong VH, Lin JC, et al. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res. 1997;12(1):111–8. https://doi.org/10.1359/jbmr.1997.12.1.111.
Feltrin GP, Macchi V, Saccavini C, Tosi E, Dus C, Fassina A, et al. Fractal analysis of lumbar vertebral cancellous bone architecture. Clin Anat. 2001;14(6):414–7. https://doi.org/10.1002/ca.1076.
Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2013;25(9):1897–904.
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–9. https://doi.org/10.1359/jbmr.080216.
Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94(1):25–34. https://doi.org/10.1007/s00223-013-9774-y.
Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29(10):1774–85. https://doi.org/10.1038/emboj.2010.60.
Baldwin KM, Haddad F. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil. 2002;81(Supplement):S40–51. https://doi.org/10.1097/00002060-200211001-00006.
Karakelides H, Nair KS. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123–48. https://doi.org/10.1016/S0070-2153(05)68005-2.
Ellman R, Grasso DJ, van Vliet M, Brooks DJ, Spatz JM, Conlon C, et al. Combined effects of botulinum toxin injection and hind limb unloading on bone and muscle. Calcif Tissue Int. 2014;94(3):327–37. https://doi.org/10.1007/s00223-013-9814-7.
Funding
This work was supported in part by ESA (ERISTO) grant (to M.G.), by MIUR grant ex60% (to M.G.) and by SIOMMMS grant (to G.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
G. Colaianni, G. Brunetti, S.C. Colucci, and M. Grano are the names of inventors of the Italian patent (MI2015A000558) and the European patent (16165324.1-1453) related to the work described.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This animal interventional study is in accordance with the European Law Implementation of Directive 2010/63/EU and all experimental protocols were reviewed and approved by the Veterinary Department of the Italian Ministry of Health (Project 522-2016PR).
Informed Consent
Not applicable. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Colaianni, G., Brunetti, G., Colucci, S.C. et al. Myokine—Irisin—and Its Effects Linking Bone and Muscle Function. Clinic Rev Bone Miner Metab 16, 16–21 (2018). https://doi.org/10.1007/s12018-017-9240-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-017-9240-x