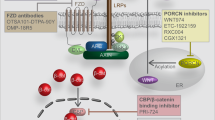
Abstract
Purpose of Review
Review current understanding of both canonical and non-canonical Wnt signaling in cancer and provide updated knowledge in current clinical trials of Wnt signaling drugs.
Recent Findings
Important roles of both canonical and non-canonical Wnt signaling in cancer have been increasingly recognized. Recent clinical trials of several Wnt-signaling drugs have showed promising outcomes. In addition, some drugs that were originally approved for the treatment of other diseases have been recently found to block Wnt signaling, highlighting their potential to treat Wnt-dependent cancer.
Summary
Dysfunction of Wnt signaling is implicated in cancer, and targeting Wnt signaling represents a useful approach to treat cancer. Current clinical trials of Wnt signaling drugs have showed promising outcomes, and repurposing the previously approved drugs for other diseases to treat Wnt-dependent cancer requires further studies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109.
Baker NE. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987;6(6):1765–73.
Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55(4):619–25.
Sharma RP. Wingless a new mutant in Drosophila melanogaster. Drosophila Inf Serv. 1973;50:134.
Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11(17):R713–24.
Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80.
De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1(3):171–81.
Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810.
Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50.
de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95(15):8847–51.
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26.
Berwick DC, Harvey K. The importance of Wnt signalling for neurodegeneration in Parkinson’s disease. Biochem Soc Trans. 2012;40(5):1123–8.
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387.
Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Chapter 2—new insights into the mechanism of Wnt signaling pathway activation. In: Jeon KW, editor. International review of cell and molecular biology, vol. 291. Cambridge: Academic Press; 2011. p. 21–71.
Schubert M, Holland L. The Wnt gene family and the evolutionary conservation of Wnt expression.In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. https://www.ncbi.nlm.nih.gov/books/NBK6212
Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5(1):a007898.
Liu C, Li Y, Semenov M, Han C, Baeg G-H, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–47.
Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13(1):149–56.
Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767.
MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26.
Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129(6):1051–63.
• Martinez S, Scerbo P, Giordano M, Daulat AM, Lhoumeau AC, Thome V, et al. The PTK7 and ROR2 protein receptors interact in the vertebrate WNT/planar cell polarity (PCP) pathway. J Biol Chem. 2015;290(51):30562–72 This study revealed a novel molecular mechanism of action of PTK7 in non-canonical WNT/PCP signaling that may promote cell and tissue movements.
Gómez-Orte E, Sáenz-Narciso B, Moreno S, Cabello J. Multiple functions of the noncanonical Wnt pathway. Trends Genet. 2013;29(9):545–53.
Sugimura R, Li L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res Part C: Embryo Today: Rev. 2010;90(4):243–56.
Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–77.
Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38(3):439–46.
Huang C-l, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, et al. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor—an expression in non-small-cell lung cancer. J Clin Oncol. 2005;23(34):8765–73.
De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin. 2011;43(10):745–56.
Slusarski DC, Pelegri F. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev Biol. 2007;307(1):1–13.
Chiurillo MA. Role of the Wnt/β-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med. 2015;5(2):84–102.
Grainger S, Traver D, Willert K. Chapter eleven—Wnt signaling in hematological malignancies. In: Larraín J, Olivares G, editors. Progress in molecular biology and translational science, vol. 153. Cambridge: Academic Press; 2018. p. 321–41.
Rapp J, Jaromi L, Kvell K, Miskei G, Pongracz JE. WNT signaling—lung cancer is no exception. Respir Res. 2017;18:167.
Tan SH, Barker N. Chapter two—Wnt signaling in adult epithelial stem cells and cancer. In: Larraín J, Olivares G, editors. Progress in molecular biology and translational science, vol. 153. Cambridge: Academic Press; 2018. p. 21–79.
Turashvili G, Bouchal J, Burkadze G, Kolar Z. Wnt Signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73(5):213–23.
Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4(5):a008052.
Tai D, Wells K, Arcaroli J, Vanderbilt C, Aisner DL, Messersmith WA, et al. Targeting the WNT signaling pathway in cancer therapeutics. Oncologist. 2015;20(10):1189–98.
Klarmann GJ, Decker A, Farrar WL. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics. 2008;3(2):59–63.
Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 2009;11(3):R32.
Valencia A, Roman-Gomez J, Cervera J, Such E, Barragan E, Bolufer P, et al. Wnt signaling pathway is epigenetically regulated by methylation of Wnt antagonists in acute myeloid leukemia. Leukemia. 2009;23(9):1658–66.
Luga V, Zhang L, Viloria-Petit Alicia M, Ogunjimi Abiodun A, Inanlou Mohammad R, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56.
Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66(21):10439.
Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1(3):279–88.
Voloshanenko O, Schwartz U, Kranz D, Rauscher B, Linnebacher M, Augustin I, et al. β-Catenin-independent regulation of Wnt target genes by RoR2 and ATF2/ATF4 in colon cancer cells. Sci Rep. 2018;8(1):3178.
Anastas JN, Biechele TL, Robitaille M, Muster J, Allison KH, Angers S, et al. A protein complex of SCRIB, NOS1AP and VANGL1 regulates cell polarity and migration, and is associated with breast cancer progression. Oncogene. 2012;31(32):3696–708.
Corda G, Sala A. Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogenesis. 2017;6(7):e364.
O'Cearbhaill RE, McMeekin DS, Mantia-Smaldone G, Gunderson C, Sabbatini P, Cattaruzza F, et al. Phase 1b of WNT inhibitor ipafricept (IPA, decoy receptor for WNT ligands) with carboplatin (C) and paclitaxel (P) in recurrent platinum-sensitive ovarian cancer (OC). J Clin Oncol. 2016;34(15_suppl):2515.
• Mita MM, Becerra C, Richards DA, Mita AC, Shagisultanova E, Osborne CRC, et al. Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with paclitaxel (P) in patients (pts) with 1st- to 3rd-line metastatic HER2-negative breast cancer (BC). J Clin Oncol. 2016;34(15_suppl):2516 This article reported the outcomes of phase 1b study of human monoclonal antibody vantictumab in combination with paclitaxel to treat patients with metastatic HER2-negative breast cancer.
Messersmith W, Cohen S, Shahda S, Lenz HJ, Weekes C, Dotan E, et al. Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with nab-paclitaxel (Nab-P) and gemcitabine (G) in patients (pts) with previously untreated stage IV pancreatic cancer (PC). Ann Oncol. 2016;27(suppl_6):677P P.
Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets. 2003;3(3):193–203.
• Ng M, Tan DSP, Subbiah V, Weekes CD, Teneggi V, Diermayr V, et al. First-in-human phase 1 study of ETC-159 an oral PORCN inhbitor in patients with advanced solid tumours. J Clin Oncol. 2017;35(15_suppl):2584 This abstract reported provisional results of the first-in-human application of small molecule inhibitor ETC-159 in an ongoing phase 1A/B trial in patients with advanced solid tumors among various cancers.
• Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci. 2013;110(50):20224 This paper reported the discovery of LGK974, a potent and specific small-molecule porcupine inhibitor and showed that LGK974 is potent and efficacious in multiple tumor models at well-tolerated doses in vivo, including murine and rat breast cancer models and a human head and neck squamous cell carcinoma model (HN30).
Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60.
Ko AH, Chiorean EG, Kwak EL, Lenz H-J, Nadler PI, Wood DL, et al. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J Clin Oncol. 2016;34(15_suppl):e15721-e.
Yoon S-S, Manasanch EE, Min CK, Kim JS, Hauptschein RS, Choi J, et al. Novel phase 1a/1b dose-finding study design of CWP232291 (CWP291) in relapsed or refractory myeloma (MM). J Clin Oncol. 2017;35(15_suppl):TPS8058-TPS.
Cortes JE, Faderl S, Pagel J, Jung CW, Yoon S-S, Koh Y, et al. Phase 1 study of CWP232291 in relapsed/refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). J Clin Oncol. 2015;33(15_suppl):7044.
• Zhang XQ, Hao JJ. Development of anticancer agents targeting the Wnt/beta-catenin signaling. Am J Cancer Res. 2015;5(8):2344–60 This is a systematical review of the potential therapeutic agents that have been developed to date for inhibition of the Wnt/β-catenin cascade.
Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11(6):791–801.
Kyle D, Proffitt DMV. Precise regulation of porcupine activity is required for physiological Wnt signaling. J Biol Chem. 2012;287:34167–78.
Madan B, Ke Z, Harmston N, Petretto E, Hill J, Keller TH, et al. Abstract B13: ETC-159 is a novel PORCN inhibitor effective for treatment of Wnt-addicted genetically defined cancers. Mol Cancer Res. 2016;14(4 Supplement):B13.
He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, et al. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6(1):7–14.
• Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11 This article reviews the preclinical data of OMP-54F28 and ongoing clinical trials of a phase 1a study and three phase 1b studies of OMP-54F28 in advanced solid tumors.
• Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. 2017;23(24):7490–7 This article reported the detailed outcome of phase 1a dose escalation study of OMP-54F28 in human patients with solid tumors.
Smith DC, Gordon M, Messersmith W, Chugh R, Mendelson D, Dupont J, et al. A first-in-human phase 1 study of anti-cancer stem cell (CSC) agent OMP-54F28 (FZD8-Fc) targeting the WNT pathway in patients with advanced solid tumors. Mol Cancer Ther. 2013;12(11):B79.
Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109(29):11717–22.
Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci U S A. 2004;101(34):12682–7.
Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105(9):1087–92.
El-Khoueiry AB, Ning Y, Yang DY, Cole S, Kahn M, Zoghbi M, et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. J Clin Oncol. 2013;31(15):a2501.
Molnar J, Somberg JC. The clinical pharmacology of ethacrynic acid. Am J Ther. 2009;16(1):86-92.
Al-Dali AM, Weiher H, Schmidt-Wolf IGH. Utilizing ethacrynic acid and ciclopirox olamine in liver cancer. Oncol Lett. 2018;16(5):6854–60.
Ren Y, Tao J, Jiang Z, Guo D, Tang J. Pimozide suppresses colorectal cancer via inhibition of Wnt/β-catenin signaling pathway. Life Sci. 2018;209:267–73.
Steinbach G, Lynch PM, Phillips RKS, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52.
Huang C, Chen Y, Liu H, Yang J, Song X, Zhao J, et al. Celecoxib targets breast cancer stem cells by inhibiting the synthesis of prostaglandin E(2) and down-regulating the Wnt pathway activity. Oncotarget. 2017;8(70):115254–69.
Egashira I, Takahashi-Yanaga F, Nishida R, Arioka M, Igawa K, Tomooka K, et al. Celecoxib and 2,5-dimethylcelecoxib inhibit intestinal cancer growth by suppressing the Wnt/β-catenin signaling pathway. Cancer Sci. 2017;108(1):108–15.
Xu L, Zhang L, Hu C, Liang S, Fei X, Yan N, et al. WNT pathway inhibitor pyrvinium pamoate inhibits the self-renewal and metastasis of breast cancer stem cells. 2016.
Xu F, Zhu Y, Lu Y, Yu Z, Zhong J, Li Y, et al. Anthelmintic pyrvinium pamoate blocks Wnt/β-catenin and induces apoptosis in multiple myeloma cells. Oncol Lett. 2018;15(4):5871–8.
Zheng L, Liu Y, Pan J. Inhibitory effect of pyrvinium pamoate on uveal melanoma cells involves blocking of Wnt/β-catenin pathway. Acta Biochim Biophys Sin. 2017;49(10):890–8.
Zhang C, Zhang Z, Zhang S, Wang W, Hu P. Targeting of Wnt/β-catenin by anthelmintic drug pyrvinium enhances sensitivity of ovarian cancer cells to chemotherapy. Med Sci Monit. 2017;23:266–75.
Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101(3):635–9.
Nugent KP, Farmer KCR, Spigelman AD, Williams CB, Phillips RKS. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. BJS. 1993;80(12):1618–9.
Tai W-P, Hu P-J, Wu J, Lin X-C. The inhibition of Wnt/β-catenin signaling pathway in human colon cancer cells by sulindac. Tumori J. 2014;100(1):97–101.
Arend RC, Londoño-Joshi AI, Samant RS, Li Y, Conner M, Hidalgo B, et al. Inhibition of Wnt/β-catenin pathway by niclosamide: a therapeutic target for ovarian cancer. Gynecol Oncol. 2014;134(1):112–20.
Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin pathway. PLOS ONE. 2011;6(12):e29290.
• Monin MB, Krause P, Stelling R, Bocuk D, Niebert S, Klemm F, et al. The anthelmintic niclosamide inhibits colorectal cancer cell lines via modulation of the canonical and noncanonical Wnt signaling pathway. J Surg Res. 2016;203(1):193–205 This article reported that niclosamide effectively inhibits colorectal cancer cell proliferation in vitro and attenuates colorectal cancer growth in a rodent model, highlighing the potential of niclosamide as a repurposed therapuetic agent in Wnt-dependpend cancer.
Osada T, Chen M, Yang XY, Spasojevic I, Vandeusen JB, Hsu D, et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011;71(12):4172.
Lu D, Liu JX, Endo T, Zhou H, Yao S, Willert K, et al. Ethacrynic acid exhibits selective toxicity to chronic lymphocytic leukemia cells by inhibition of the Wnt/beta-catenin pathway. PLoS One. 2009;4(12):e8294-e.
Wu W, Zhu H, Fu Y, Shen W, Miao K, Hong M, et al. High LEF1 expression predicts adverse prognosis in chronic lymphocytic leukemia and may be targeted by ethacrynic acid. Oncotarget. 2016;7(16):21631–43.
Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1 alpha. Nat Chem Biol. 2010;6(11):829–36.
Venerando A, Girardi C, Ruzzene M, Pinna LA. Pyrvinium pamoate does not activate protein kinase CK1, but promotes Akt/PKB down-regulation and GSK3 activation. Biochem J. 2013;452(1):131.
• Fako V, Yu Z, Henrich CJ, Ransom T, Budhu AS, Wang XW. Inhibition of wnt/beta-catenin signaling in hepatocellular carcinoma by an antipsychotic drug pimozide. Int J Biol Sci. 2016;12(7):768–75 This article reported a novel application of antipsychotic agent pimozide as a potential therapeutic targeting the Wnt/β-catenin signaling pathway against Hep3B and HepG2 HCC cells.
Fidahic M, Jelicic Kadic A, Radic M, Puljak L. (2017). “Celecoxib for rheumatoid arthritis.” Cochrane Database of Syst Rev (6).
Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the class study: a randomized controlled trial. JAMA. 2000;284(10):1247–55.
Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA. 1999;282(20):1921–8.
Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci. 1998;95(22):13313.
Han A, Song Z, Tong C, Hu D, Bi X, Augenlicht LH, et al. Sulindac suppresses beta-catenin expression in human cancer cells. Eur J Pharmacol. 2008;583(1):26–31.
Boon EM, Keller JJ, Wormhoudt TA, Giardiello FM, Offerhaus GJ, van der Neut R, et al. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90(1):224–9.
Stein U, Arlt F, Smith J, Sack U, Herrmann P, Walther W, et al. Intervening in beta-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia. 2011;13(2):131–44.
Chen M, Wang J, Lu J, Bond MC, Ren X-R, Lyerly HK, et al. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry. 2009;48(43):10267–74.
Funding
This work was supported by start-up funds from Western University of Health Sciences to J.H.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evolving Therapies
Rights and permissions
About this article
Cite this article
Harb, J., Lin, PJ. & Hao, J. Recent Development of Wnt Signaling Pathway Inhibitors for Cancer Therapeutics. Curr Oncol Rep 21, 12 (2019). https://doi.org/10.1007/s11912-019-0763-9
Published:
DOI: https://doi.org/10.1007/s11912-019-0763-9