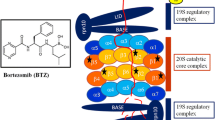
Abstract
Proteasome inhibition has a validated role in cancer therapy since the successful introduction of bortezomib for the treatment of multiple myeloma (MM) and mantle cell lymphoma, leading to the development of second-generation proteasome inhibitors (PI) for MM patients in whom currently approved therapies have failed. Five PIs have reached clinical evaluation, with the goals of improving efficacy and limiting toxicity, including peripheral neuropathy (PN). Carfilzomib, an epoxyketone with specific chymothrypsin-like activity, acts as an irreversible inhibitor and was recently FDA approved for the response benefit seen in relapsed and refractory MM patients previously treated with bortezomib, thalidomide and lenalidomide. ONX-0912 is now under evaluation as an oral form with similar activity. The boronate peptides MLN9708 and CEP-18770 are orally bioactive bortezomib analogs with prolonged activity and greater tissue penetration. NPI-0052 (marizomib) is a unique, beta-lactone non-selective PI that has been shown to potently overcome bortezomib resistance in vitro. All of these second-generation PIs demonstrate encouraging anti-MM activity and appear to reduce the incidence of PN, with clinical trials ongoing.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Laubach JP, Mahindra A, Mitsiades CS, et al. The use of novel agents in the treatment of relapsed and refractory multiple myeloma. Leukemia. 2009;23(12):2222–32.
Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Proteasome inhibition in the treatment of cancer. Cell Cycle. 2005;4(2):290–6.
Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23(4):667–75.
Kirk CJ. Discovery and development of second-generation proteasome inhibitors. Semin Hematol. 2012;49(3):207–14.
•• Moreau P, Richardson PG, Cavo M, et al. Proteasome inhibitors in multiple myeloma: ten years later. Blood. 2012 May 29. A comprehensive review of PIs, both old and new.
Kuhn DJ, Orlowski RZ. The immunoproteasome as a target in hematologic malignancies. Semin Hematol. 2012;49(3):258–62.
Chauhan D, Tian Z, Nicholson B, et al. Deubiquitylating enzyme USP-7, a novel therapeutic target in multiple myeloma. ASH Annual Meeting Abstracts. 2009;114(22):610.
• Kuhn DJ, Hunsucker SA, Chen Q, et al. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood. 2009;113(19):4667–76. An important preclinical study of immunoproteasome inhibition in MM.
Roccaro AM, Sacco A, Aujay M, et al. Selective inhibition of chymotrypsin-like activity of the immunoproteasome and constitutive proteasome in Waldenstrom macroglobulinemia. Blood. 2010;115(20):4051–60.
Kupperman E, Lee EC, Cao Y, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70(5):1970–80.
Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8(5):407–19.
Dick LR, Fleming PE. Building on bortezomib: second-generation proteasome inhibitors as anti-cancer therapy. Drug Discov Today. 2010;15(5–6):243–9.
• Chauhan D, Tian Z, Zhou B, et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res. 2011;17(16):5311–21. An important preclinical study evaluating the activity of MLN9708 in MM.
Piva R, Ruggeri B, Williams M, et al. CEP-18770: a novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood. 2008;111(5):2765–75.
Lee EC, Fitzgerald M, Bannerman B, et al. Antitumor activity of the investigational proteasome inhibitor MLN9708 in mouse models of B-cell and plasma cell malignancies. Clin Cancer Res. 2011;17(23):7313–23.
Bacco AD, Berger A, Gupta N, et al. Tumor drug distribution and target engagement of MLN9708, an investigational proteasome inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30(suppl):abstr 3077.
Sanchez E, Li M, Steinberg JA, et al. The proteasome inhibitor CEP-18770 enhances the anti-myeloma activity of bortezomib and melphalan. Br J Haematol. 2010;148(4):569–81.
Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110(9):3281–90.
Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–91.
• Chauhan D, Singh AV, Aujay M, et al. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood. 2010;116(23):4906–15. An important preclinical study of ONX 0912 demonstrating activity in MM.
Hurchla MA, Garcia-Gomez A, Hornick MC, et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia. 2012;Jul 5.
Yang J, Wang Z, Fang Y, et al. Pharmacokinetics, pharmacodynamics, metabolism, distribution, and excretion of carfilzomib in rats. Drug Metab Dispos. 2011;39(10):1873–82.
Arastu-Kapur S, Anderl JL, Kraus M, et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17(9):2734–43.
Dasmahapatra G, Lembersky D, Son MP, et al. Carfilzomib interacts synergistically with histone deacetylase inhibitors in mantle cell lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2011;10(9):1686–97.
Zhou HJ, Aujay MA, Bennett MK, et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047). J Med Chem. 2009;52(9):3028–38.
Singh AV, Palladino MA, Lloyd GK, et al. Pharmacodynamic and efficacy studies of the novel proteasome inhibitor NPI-0052 (marizomib) in a human plasmacytoma xenograft murine model. Br J Haematol. 2010;149(4):550–9.
Potts BC, Albitar MX, Anderson KC, et al. Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11(3):254–84.
Kraus M, Florea B, Bader J, et al. Selective inhibition of the proteasome's {beta}2 catalytic subunit alone does not induce cytotoxicity, but resensitizes bortezomib-refractory myeloma cells for bortezomib treatment. ASH Annual Meeting Abstracts. 2011;118(21):2915.
Chauhan D, Singh A, Brahmandam M, et al. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111(3):1654–64.
•• Chauhan D, Singh AV, Ciccarelli B, et al. Combination of novel proteasome inhibitor NPI-0052 and lenalidomide trigger in vitro and in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2010;115(4):834–45. A study describing the potent combinational effect of lenalidomide and NPI-0052 and showing synergy preclinically against MM.
Mitsiades CS, Hideshima T, Chauhan D, et al. Emerging treatments for multiple myeloma: beyond immunomodulatory drugs and bortezomib. Semin Hematol. 2009;46(2):166–75.
Ahn KS, Sethi G, Chao TH, et al. Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-kappaB regulated gene products. Blood. 2007;110(7):2286–95.
US National Institute of Health. ClinicalTrial.gov. 2012 [cited 2012 July 31]; Available from: http://clinicaltrials.gov/.
O'Connor OA, Stewart AK, Vallone M, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15(22):7085–91.
Alsina M, Trudel S, Vallone M, et al. Phase 1 single agent antitumor activity of twice weekly consecutive day dosing of the proteasome inhibitor carfilzomib (PR-171) in hematologic malignancies. ASH Annual Meeting Abstracts. 2007;110(11):411.
• Alsina M, Trudel S, Furman RR, et al. A phase 1 single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;Jul 3. This important phase I study of carfilzomib in relapsed/refractory MM established the dose and schedule of carfilzomib in this setting.
Squifflet P, Michiels S, Siegel DS, et al. Multivariate modelling reveals evidence of a dose-response relationship in phase 2 studies of single-agent carfilzomib. ASH Annual Meeting Abstracts. 2011;118(21):1877.
Papadopoulos KP, Lee P, Singhal S, et al. A phase 1b/2 study of prolonged infusion carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma: updated efficacy and tolerability from the completed 20/56 mg/m2 expansion cohort of PX-171-007. ASH Annual Meeting Abstracts. 2011;118(21):2930.
Singhal S, Siegel DS, Martin T, et al. Integrated safety from phase 2 studies of monotherapy carfilzomib in patients with relapsed and refractory Multiple Myeloma (MM): an updated analysis. ASH Annual Meeting Abstracts. 2011;118(21):1876.
•• Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;Jul 25. This pivotal phase II study of carfilzomib in relapsed/refractory MM demonstrated response benefit in this heavily pretreated, advanced patient population.
Vij R, Wang M, Kaufman JL, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119(24):5661–70.
Wang L, Siegel DS, Jakubowiak AJ, et al. The speed of response to single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma: an exploratory analysis of results from 2 multicenter phase 2 clinical trials. ASH Annual Meeting Abstracts. 2011;118(21):3969.
Berenson JR, Yellin O, Dichmann R, et al. A phase I/II study of carfilzomib (CFZ) as a replacement for bortezomib (BTZ) for multiple myeloma (MM) patients (Pts) progressing while receiving a BTZ-containing combination regimen. J Clin Oncol. 2012;30(suppl):abstr 8098.
•• Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012. This seminal study of carfilzomib, lenalidomide, and dexamethasone in newly diagnosed MM showed remarkable activity (ORR of 94 %) and favorable tolerability, with a low overall rate of treatment –emergent peripheral neuropathy (23 %).
Sonneveld P, Hacker E, Zweegman S, et al. Carfilzomib Combined with Thalidomide and Dexamethasone (CARTHADEX) as induction treatment prior to High-Dose Melphalan (HDM) in newly diagnosed patients with Multiple Myeloma (MM). A Trial of the European Myeloma Network EMN. ASH Annual Meeting Abstracts. 2011;118(21):633.
Mikhael J, Reeder CB, Libby EN, et al. A phase I/II trial of cyclophosphamide, carfilzomib, thalidomide, and dexamethasone (CYCLONE) in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2012;30(suppl):abstr 8010.
Kolb B, Hulin C, Caillot D, et al. Phase I/II study of carfilzomib plus melphalan-prednisone (CMP) in elderly patients with de novo multiple myeloma. J Clin Oncol. 2012;30(suppl):abstr 8009.
European Medicines Agency. EU Clinical Trials Register. 2012 [cited 2012 July 31]; Available from: https://www.clinicaltrialsregister.eu.
Gupta N, Saleh M, Venkatakrishnan K. Flat-dosing versus BSA-based dosing for MLN9708, an investigational proteasome inhibitor: Population Pharmacokinetic (PK) analysis of pooled data from 4 phase-1 studies. ASH Annual Meeting Abstracts. 2011;118(21):1433.
Kumar S, Bensinger W, Reeder CB, et al. Weekly dosing of the investigational oral proteasome inhibitor MLN9708 in patients (pts) with relapsed/refractory multiple myeloma (MM): A phase I study. J Clin Oncol. 2012;30(suppl):abstr 8034.
Lonial S, Baz RC, Wang M, et al. Phase I study of twice-weekly dosing of the investigational oral proteasome inhibitor MLN9708 in patients (pts) with relapsed and/or refractory multiple myeloma (MM). J Clin Oncol. 2012;30(suppl):abstr 8017.
Martin P, Chang JE, Rifkin RM, et al. MLN9708, an investigational proteasome inhibitor, in patients (pts) with relapsed/refractory lymphoma: Emerging data from a phase I dose-escalation study. J Clin Oncol. 2012 30(suppl):abstr 8064.
Smith DC, Sullivan D, Infante JR, et al. MLN9708, an investigational proteasome inhibitor, in patients (pts) with solid tumors: Updated phase I results. J Clin Oncol. 2012;30:suppl; abstr e13603.
•• Richardson PG, Berdeja JG, Niesvizky R, et al. Oral weekly MLN9708, an investigational proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma (MM): A phase I/II study. J Clin Oncol. 2012;30(suppl):abstr 8033. This encouraging clinical study of MLN9708 in combination with lenalidomide and dexamethasone as an all oral regimen in newly diagnosed MM, demonstrated 100 % ORR and favorable tolerability.
Hofmeister CC, Richardson P, Zimmerman T, et al. Clinical trial of the novel structure proteasome inhibitor NPI-0052 in patients with relapsed and relapsed/refractory multiple myeloma (r/r MM). J Clin Oncol. 2009;27(15s):suppl; abstr 8505.
Spencer A, Millward M, Mainwaring P, et al. Phase 1 clinical trial of the novel structure proteasome inhibitor NPI-0052. ASH Annual Meeting Abstracts. 2009;114(22):2693.
Richardson PG, Spencer A, Cannell P, et al. Phase 1 clinical evaluation of twice-weekly marizomib (NPI-0052), a novel proteasome inhibitor, in patients with relapsed/refractory Multiple Myeloma (MM). ASH Annual Meeting Abstracts. 2011;118(21):302.
Millward M, Price T, Townsend A, et al. Phase 1 clinical trial of the novel proteasome inhibitor marizomib with the histone deacetylase inhibitor vorinostat in patients with melanoma, pancreatic and lung cancer based on in vitro assessments of the combination. Invest New Drugs. 2011 Nov 12.
Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23):4745–53.
Papadopoulos KP, Mendelson DS, Tolcher AW, et al. A phase I, open-label, dose-escalation study of the novel oral proteasome inhibitor (PI) ONX 0912 in patients with advanced refractory or recurrent solid tumors. J Clin Oncol. 2011;29:suppl; abstr 3075.
Disclosures
P. Lawasut, D.Chauhan, J. Laubach, C. Hayes, C. Fabre, and M. Maglio declare no potential conflicts of interest relevant to this article.
T. Hideshima is a consultant for Acetylon Pharmaceuticals.
C. Mitsiades has been a consultant for Millennium, Celgene, Novartis, Bristol-Myers Squibb, Merck &Co., Centocor, and Arno Therapeutics, and has received research funding from Amgen, AVEO Pharma, OSI, EMD Serono, Sunesis, Genzyme, and Johnson & Johnson.
K. Anderson is a member of advisory committees for Millennium, Nereus, Onyx, Johnson & Johnson, and Celgene Corporation, and is a founder of Acetylon Pharmaceuticals.
P. Richardson is a member of advisory committees for Millennium, Nereus, Onyx, Johnson & Johnson and Celgene Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawasut, P., Chauhan, D., Laubach, J. et al. New Proteasome Inhibitors in Myeloma. Curr Hematol Malig Rep 7, 258–266 (2012). https://doi.org/10.1007/s11899-012-0141-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-012-0141-2