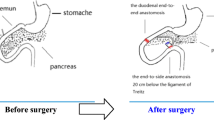
Abstract
Background
This study aimed to investigate the influence of new biliopancreatic diversion (NBPD) and duodenal-jejunal bypass (DJB) surgery on blood glucose, lipids, gastrointestinal hormones, and insulin in Goto-Kakizaki (GK) rats, an animal model for type 2 diabetes, in order to elucidate the mechanisms underlying the therapeutic effect of these types of surgery on this clinical condition.
Methods
Thirty 30 male GK rats (SPF) aged 12 weeks were randomly assigned into three groups (n = 10 per group): sham group, NBPD group, and DJB group. Body weight, random plasma glucose, fasting plasma glucose (FPG), oral glucose tolerance (OGT), blood lipids, plasma insulin, glucagon like peptide-1 (GLP-1), and gastric inhibitory polypeptide (GIP) were measured before and after surgery.
Results
NBPD surgery improved glucose tolerance, decreased fasting free fatty acids, triglycerides, and cholesterol. It also increased fasting and postprandial GIP, but caused no change in GLP-1. DJB surgery produced results similar to NBPD surgery except for causing a decrease in postprandial GLP-1 and insulin, and a larger increase in fasting GIP.
Conclusions
Moving the biliopancreatic duct outlet to the mid-jejunum (NBPD surgery) improves glucose tolerance and increases GIP, but does not change GLP-1. Adding duodenal bypass (DJB surgery) increases fasting GIP and decreases postprandial GLP-1.





Similar content being viewed by others
References
Rubino F, Marescaux J. Effect of duodenal–jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11.
Alleotti E, Palma RT, Pinto Jr PE, et al. Biliopancreatic diversion with duodenojenunal exclusion associated with truncal vagotomy. A new proposal for type 2 diabetes mellitus treatment. Acta Cirirgica Brasil. 2012;27:577–84.
Koehestanie P, Dogan K, Berends F, et al. Duodenal-jejunal bypass liner implantation provokes rapid weight loss and improved glycemic control, accompanied by elevated fasting ghrelin levels. Endosc Int Open. 2014. doi:10.1055/s-0034-1365222.
Raffaelli M, Iaconelli A, Nanni G, et al. Effects of biliopancreatic diversion on diurnal leptin, insulin and free fatty acid levels. BJS. 2015;102:682–90.
Gan SS, Talbot ML, Jorgensen JO. Efficacy of surgery in the management of obesity-related type 2 diabetes mellitus. ANZ J Surg. 2007;77(11):958–62.
Pereferrer FS, Gonzalez MH, Rovira AF, et al. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18(1):97–108.
American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33:692.
Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61.
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52.
Akash MSH, Rehman K, Chen S. Goto-kakizaki rats: Suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr Diabetes Rev. 2013;9:1–10.
Weng S-G. Modified biliopancreatic diversion for GK rats: a proposal for a simpler technique and mechanism research. Obes Surg. 2012;22:997–8.
Weng SG, Zhang B, Feng S, et al. Effects of modified biliopancreatic diversion on glucose tolerance of GK rats. Obes Surg. 2012;23:522–30.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57.
Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–31.
Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7.
Young AA, Gedulin B, Vine W, et al. Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia. 1995;38:642–8.
Han H, Wang L, Du H, et al. Expedited biliopancreatic juice flow to the distal gut benefits the diabetes control after duodenal jejunal bypass. Obes Surg. 2015;25:1802–9.
Zhang GY, Wang TT, Cheng ZQ, et al. Resolution of diabetes mellitus by ileal transposition compared with bilioancreatic diversion in a nonobese animal model of diabetes. Can J Surg. 2011;54:243–51.
Patriti A, Aisa M, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Speck M, Cho YM, Asadi A, et al. Duodenal-jejunal bypass protects GK rats from b-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300:E923–32.
Charles MA, Eschwege E, Thibult N, et al. The role of NEFA in the deterioration of glucose tolerance in Caucasian subjects:result of the pairs prospective study. Diabetologia. 1997;40:1101–9.
Taskinen MR, Bogardus C, Kennedy A, et al. Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest. 1985;76(2):637–44.
Boden G. Does inhibition of beta cell proliferation by free fatty acid in mice explain the progressive failure of insulin secretion in type 2 diabetes? Diabetes. 2012;61(3):560–1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of informed consent
Not applicable
Statement of human and animal rights
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Supported by: The Fujian Provincial Natural Science Foundation, No. 2014J01312. Training Project of Young Talents in Health System of Fujian Province, No.2014-ZQN-ZD-17. Professor Development Fund of Fujian Medical University, No.JS14020.
Rights and permissions
About this article
Cite this article
Weng, S., Zhang, B., Xu, C. et al. Influence of New Modified Biliopancreatic Diversion on Blood Glucose and Lipids in GK rats. OBES SURG 27, 657–664 (2017). https://doi.org/10.1007/s11695-016-2320-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2320-z