Abstract
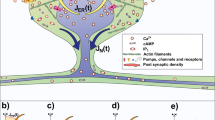
We study the dynamics of a model of membrane vesicle transport into dendritic spines, which are bulbous intracellular compartments in neurons driven by molecular motors. We reduce the lubrication model proposed in Fai et al. (Phys Rev Fluids 2:113601, 2017) to a fast–slow system, yielding an analytically and numerically tractable equation equivalent to the original model in the overdamped limit. The model’s key parameters include: (1) the ratio of motors that prefer to push toward the head of the dendritic spine to the motors that prefer to push in the opposite direction, and (2) the viscous drag exerted on the vesicle by the spine constriction. We perform a numerical bifurcation analysis in these parameters and find that steady-state vesicle velocities appear and disappear through several saddle-node bifurcations. This process allows us to identify the region of parameter space in which multiple stable velocities exist. We show by direct calculations that there can only be unidirectional motion for sufficiently close vesicle-to-spine diameter ratios. Our analysis predicts the critical vesicle-to-spine diameter ratio, at which there is a transition from unidirectional to bidirectional motion, consistent with experimental observations of vesicle trajectories in the literature.








Similar content being viewed by others
References
Acheson DJ (1991) Elementary fluid dynamics
Adrian M, Kusters R, Wierenga CJ, Storm C, Hoogenraad CC, Kapitein LC (2014) Barriers in the brain: resolving dendritic spine morphology and compartmentalization. Front Neuroanat 8:142
Allard J, Doumic M, Mogilner A, Oelz D (2019) Bidirectional sliding of two parallel microtubules generated by multiple identical motors. J Math Biol 72:1–24
Arellano JI, Benavides-Piccione R, DeFelipe J, Yuste R (2007) Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci 1:10
Bagnall JS, Byun S, Begum S, Miyamoto DT, Hecht VC, Maheswaran S, Stott SL, Toner M, Hynes RO, Manalis SR (2015) Deformability of tumor cells versus blood cells. Sci Rep 5:18542
Bell GI (1978) Models for the specific adhesion of cells to cells. Science 200(4342):618–627
Berger DR, Sebastian Seung H, Lichtman JW (2018) Vast (volume annotation and segmentation tool): efficient manual and semi-automatic labeling of large 3d image stacks. Front Neural Circuits 12:88
Bressloff P, Newby J (2009) Directed intermittent search for hidden targets. New J Phys 11(2):023033
Bressloff PC, Newby JM (2013) Metastability in a stochastic neural network modeled as a velocity jump Markov process. SIAM J Appl Dyn Syst 12(3):1394–1435
Broer HW, Kaper TJ, Krupa M (2013) Geometric desingularization of a cusp singularity in slow-fast systems with applications to Zeeman’s examples. J Dyn Diff Equ 25(4):925–958
Christopher Risher W, Ustunkaya T, Alvarado JS, Eroglu C (2014) Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PloS One 9(9):e107591
Dallon JC, Leduc C, Etienne-Manneville S, Portet S (2019) Stochastic modeling reveals how motor protein and filament properties affect intermediate filament transport. J Theor Biol 464:132–148
Dawson G, Häner E, Juel A (2015) Extreme deformation of capsules and bubbles flowing through a localised constriction. Procedia IUTAM 16:22–32
Duncanson WJ, Kodger TE, Babaee S, Gonzalez G, Weitz DA, Bertoldi K (2015) Microfluidic fabrication and micromechanics of permeable and impermeable elastomeric microbubbles. Langmuir 31(11):3489–3493
Ermentrout GB (2002) Simulating, analyzing, and animating dynamical systems: a guide to XPPAUT for researchers and students, vol 14. SIAM, New Delhi
Esteves M, da Silva M, Adrian PS, Lipka J, Watanabe T, Cho S, Futai K, Wierenga CJ, Kapitein LC, Hoogenraad CC (2015) Positioning of ampa receptor-containing endosomes regulates synapse architecture. Cell Rep 13(5):933–943
Fai TG, Kusters R, Harting J, Rycroft CH, Mahadevan L (2017) Active elastohydrodynamics of vesicles in narrow blind constrictions. Phys Rev Fluids 2(11):113601
Fenichel N (1979) Geometric singular perturbation theory for ordinary differential equations. J Differ Equ 31(1):53–98
Freund JB (2013) The flow of red blood cells through a narrow spleen-like slit. Phys Fluids 25(11):110807
Gabriele S, Versaevel M, Preira P, Théodoly O (2010) A simple microfluidic method to select, isolate, and manipulate single-cells in mechanical and biochemical assays. Lab Chip 10(11):1459–1467
Gardiner CW (2009) Stochastic methods : a handbook for the natural and social sciences. Springer, Berlin ISBN 978-3-540-70712-7
Gray EG (1959) Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat 93(Pt 4):420
Guérin T, Prost J, Joanny J-F (2011) Motion reversal of molecular motor assemblies due to weak noise. Phys Rev Lett 106(6):068101
Holtmaat A, Svoboda K (2009) Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10(9):647–658
Hoppensteadt FC, Peskin CS (2012) Modeling and simulation in medicine and the life sciences, vol 10. Springer, Berlin
Jülicher F, Prost J (1995) Cooperative molecular motors. Phys Rev Lett 75(13):2618
Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J (2010) Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci 33(3):121–129
Kasthuri N, Hayworth KJ, Berger DR, Schalek RL, Conchello JA, Knowles-Barley S, Lee D, Vázquez-Reina A, Kaynig V, Jones TR et al (2015) Saturated reconstruction of a volume of neocortex. Cell 162(3):648–661
Koehnle TJ, Brown A (1999) Slow axonal transport of neurofilament protein in cultured neurons. J Cell Biol 144(3):447–458
Kunwar A, Tripathy SK, Jing X, Mattson MK, Anand P, Sigua R, Vershinin M, McKenney RJ, Clare CY, Mogilner A et al (2011) Mechanical stochastic tug-of-war models cannot explain bidirectional lipid-droplet transport. Proc Nat Acad Sci 108(47):18960–18965
Kusters R, van der Heijden T, Kaoui B, Harting J, Storm C (2014) Forced transport of deformable containers through narrow constrictions. Phys Rev E 90(3):033006
Kuznetsov YA (2013) Elements of applied bifurcation theory, vol 112. Springer, Berlin
Li Y, Sarıyer OS, Ramachandran A, Panyukov S, Rubinstein M, Kumacheva E (2015) Universal behavior of hydrogels confined to narrow capillaries. Sci Rep 5:17017
Müller MJI, Klumpp S, Lipowsky R (2008) Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc Nat Acad Sci 105(12):4609–4614
Newby J, Bressloff PC (2010) Random intermittent search and the tug-of-war model of motor-driven transport. J Stat Mech Theory Exp 2010(04):P04014
Newby JM, Bressloff PC (2009) Directed intermittent search for a hidden target on a dendritic tree. Phys Rev E 80(2):021913
Newby JM, Keener JP (2011) An asymptotic analysis of the spatially inhomogeneous velocity-jump process. Multiscale Model Simul 9(2):735–765
Nimchinsky EA, Sabatini BL, Svoboda K (2002) Structure and function of dendritic spines. Annu Rev Physiol 64(1):313–353
Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD (2006) Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52(5):817–830
Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14(3):285
Portet S, Leduc C, Etienne-Manneville S, Dallon J (2019) Deciphering the transport of elastic filaments by antagonistic motor proteins. Phys Rev E 99(4):042414
Rorai C, Touchard A, Zhu L, Brandt L (2015) Motion of an elastic capsule in a constricted microchannel. Eur Phys J E 38(5):49
Sangwon B, Sungmin S, Dario A, Nathan C, Josephine S, Ho KJ, Hecht Vivian C, Winslow Monte M, Tyler J, Parag M, Manalis Scott R (2013) Characterizing deformability and surface friction of cancer cells. Proc Natl Acad Sci 110(19):7580–7585. https://doi.org/10.1073/pnas.1218806110
Walker CL, Uchida A, Li Y, Trivedi N, Fenn JD, Monsma PC, Lariviére RC, Julien J-P, Jung P, Brown A (2019) Local acceleration of neurofilament transport at nodes of Ranvier. J Neurosci 39(4):663–677
Zhiping W, Edwards Jeffrey G, Nathan R, William PD, Ryan K, Xiang-dong L, Davison Ian G, Mitsuo I, Mercer John A, Kauer Julie A, Ehlers Michael D (2008) Myosin Vb mobilizes recycling endosomes and ampa receptors for postsynaptic plasticity. Cell 135(3):535–548. https://doi.org/10.1016/j.cell.2008.09.057
Yuste R (2010) Dendritic spines. MIT Press, Cambridge
Zimmermann D, Santos A, Kovar DR, Rock RS (2015) Actin age orchestrates myosin-5 and myosin-6 run lengths. Curr Biol 25(15):2057–2062
Zimmermann H (1996) Accumulation of synaptic vesicle proteins and cytoskeletal specializations at the peripheral node of Ranvier. Microsc Res Tech 34(5):462–473
Acknowledgements
The authors thank Chris H. Rycroft and Jonathan Touboul for reading the early version of the text and providing useful feedback.
Funding
The authors acknowledge support under the National Institute of Health grant T32 NS007292 (YP) and National Science Foundation grant DMS-1913093 (TGF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Code Availability
All code and data are available on GitHub: https://github.com/youngmp/park_fai_2020
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Numerics
In this section, we detail the numerical methods and parameters of the fast–slow lubrication model.
1.1 Integration
For convenience, we restate the original fast–slow lubrication model here:
We solve Eq. (24) numerically by taking \(\varepsilon \) nonzero and integrating forwards in time. We obtain visually indistinguishable results using either Python’s odeint routine or the forward Euler method. This approach is beneficial because velocities satisfying instantaneous force–balance, i.e., U such that \(F(U) - \zeta (Z)U=0\), are fixed points of a one-dimensional ODE (assuming Z is constant). The question of convergence is then straightforward: If an initial condition is within the basin of attraction of a stable velocity, it will converge to this velocity.
Another benefit of taking this approach is that for \(\varepsilon \) sufficiently small, system (24) is sufficiently similar to the overdamped system when \(\varepsilon =0\), as guaranteed by Fenichel theory (Fenichel 1979; Broer et al. 2013). When \(\varepsilon \ll 1\), there is a separation of timescales, and very small time steps are required for numerical stability. We find that choosing \(\varepsilon =1\) works well in practice, as the dynamics follow the slow manifold but do not require impractically small time steps. This approximation works even at moderate values of \(\varepsilon \) (e.g., at \(\varepsilon =1\)): Whereas we have non-dimensionalized velocity in terms of the free space prediction of Stokes’ law, in confined geometries, there is a small parameter which may be obtained by rescaling \(\varepsilon \) by the effective drag. For a given choice of \(\varepsilon \), we typically take the time step dt to be smaller by one or two orders of magnitude.
1.2 Continuation
General continuation strategies in one and two parameters can be found in Chapter 10.3 of Kuznetsov (Kuznetsov 2013). We use XPPAUTO (Ermentrout 2002) (version 8) to generate our bifurcation diagrams unless stated otherwise. For the generation of the one- and two-parameter diagrams, we use the following numerical values:

All other numerical values remain at default values. We adjust Ntst, Nmax, Npr, Par Min, and Par Max as needed. The displayed Dsmax value may make AUTO run multiple passes over some branches, in which case we reduce Dsmax to 0.001, preventing this issue while incrementing at a reasonable pace. We include many more details in a mini-tutorial on our GitHub repository at https://github.com/youngmp/park_fai_2020.
1.3 Cusps
Saddle nodes may be computed by finding tangencies in the right-hand side of Eq. (19) with the constraint that U satisfies
The conditions for a saddle-node bifurcation are (Kuznetsov 2013):
We denote any U that simultaneously satisfies these equations by \(U^*\). We can simplify the search for saddle nodes by writing the system
We can just as easily use \({\bar{\zeta }}\) and look for fixed points in \(({\bar{U}}, {\bar{\zeta }})\) as a function of \(\phi \). It is merely a matter of preference. We use \({\bar{U}}\) and \({\bar{\phi }}\) to emphasize the difference between (26) and the original fast subsystem (19). This new system eliminates one parameter when it comes to computing bifurcation diagrams. Fixed points of (26) correspond to saddle-node bifurcations in the fast subsystem (19). The \({\bar{U}}\) nullcline of (26) shows the stable fixed points in (19), and the \({\bar{U}}\) nullcline intersections with the \({\bar{\phi }}\) nullcline correspond to saddle nodes. Thus, the phase space of (26) corresponds to one-parameter bifurcations in (19), and the one-parameter bifurcations in (26) correspond to two-parameter bifurcations in (19). In particular, saddle-node bifurcations of (26) correspond to cusp bifurcations in (19). We exploit the latter fact to generate the diagram of cusp bifurcations (Fig. 7).
In practice, we compute intersections in the nullclines of Eq. (26) and track where the intersections change in number. Suppose that we are interested in finding cusp bifurcations as a function of \(\pi _4\) and \(\pi _5\) for a given \(\zeta \). On one side of the cusp bifurcation, Eq. (26) shows two fixed points and on the other side shows zero. By defining a function that returns \(+1\) on one side of the bifurcation and \(-1\) on the other, we can use a root-finding method such as Brentq to determine \(\pi _5\) where there is a transition in the fixed point number to high numerical precision.
Properties of the Force–Velocity Curve
The force–velocity curves are very well behaved, but it is not always obvious how. In this section, we briefly show some properties used in the text.
1.1 Continuity
The continuity of the force–velocity curve [Eq. (13)] is not immediately apparent. We rewrite the force–velocity curve verbatim for convenience:
In particular, there are two possible problem areas: when \({\widetilde{U}}=0\) and when \({\widetilde{U}}=1/\pi _6\). The latter case is especially important because numerical problems occasionally arise when evaluating \({\widetilde{U}}=1/\pi _6\) directly. In the first case, the left limit is straightforward:
The right limit depends on the behavior of the exponentials. Noting that \(\exp (-\pi _5/\pi _6{\widetilde{U}}) \rightarrow 0\) as \({\widetilde{U}}\rightarrow 0^+\), it is straightforward to check that the left and right limits agree independent of the parameters \(\pi _i\), and therefore, \({\widetilde{F}}_A\) is continuous at \({\widetilde{U}}=0\). Continuity of \({\widetilde{F}}_{-A}\) follows by definition.
In the second case, when \({\widetilde{U}}=1/\pi _6\ge 0\), we take a close look at the second line of Eq. (27). Potential problems arise in the term \((1-e^{\pi _5}e^{-\pi _5/\pi _6{\widetilde{U}}})/(1-\pi _6{\widetilde{U}})\). For convenience, let \(v:=1-1/(\pi _6 {\widetilde{U}})\) so that \(v\rightarrow 0\) as \({\widetilde{U}}\rightarrow 1/\pi _6\). Then, the term becomes
We have used a Taylor expansion of the exponential about zero. So the term converges to \(\pi _5-1\) as \(v\rightarrow 0\) on either side of the limit. It follows that the function \({\widetilde{F}}_A\) is continuous at \({\widetilde{U}}=1/\pi _6\), as desired.
1.2 Limits
Consider \(\pi _4\rightarrow 0\). This limit occurs when attachments occur at zero position (\(A=0\)), or when the force-scaling parameter \(\gamma =0\). Both cases appear to be somewhat unrealistic: In the former case, newly attached crossbridges will apply no force [Eq. (6)], and in the latter case, the force exerted by a single motor is always zero. We find that the term \(e^{\pi _4}-1\) appears in the denominator of several terms and competes with U when U is small. For \(|U|\gg 0\), the term \((e^{\pi _4}-1)^{-1}\) dominates, and we expect \(F_A(U)\rightarrow \infty \) as \(\pi _4 \rightarrow 0\). When \(|U|\ll 1\), we expect \(F_A(U)\rightarrow -1\) as \(U \rightarrow 0\) for each \(\pi _4\) small.
In contrast, the dimensional force–velocity curve converges for \(\pi _4\) small and diverges for \(\pi _4\). A non-dimensionalization using \(F_1 = F_0/(e^{\pi _4}-1)\) would remove this problem of divergence, but this rescaling is not necessary when considering bifurcations: For any fixed point \(F(U,\pi _4)=0\), where F is the non-dimensional net force, multiplying both sides by the scaling factor \((e^{\pi _4}-1)\) yields \((e^{\pi _4}-1)F(U,\pi _4)=0\), so scaling preserves fixed points as a function of \(\pi _4\).
Comparisons to the Allard et al. (2019) Symmetric Kinesin Motor Model
The choice of motor model does not qualitatively change multistability as a function of drag. Using a similar symmetric motor model of kinesin motors from Allard et al. (2019), we add an analogous constriction term in their force–balance equation and re-derive the master equation. To make direct comparisons to our results, we go a step further and derive the Fokker–Planck equation, from which we produce bifurcation diagrams. To minimize confusion, we rephrase their problem in terms of molecular motors in spines. In particular, we consider two identical species of kinesin motors, where one species prefers to push up and the other prefers to push down.
We note several important differences that allow a very straightforward derivation of the mean-field equations in the symmetric kinesin model. First, in contrast to the myosin motors considered in the present study, the kinesin motor model does not have spatial dependence. Second, in contrast to the attachment and detachment rate of myosin motors that depend on position-dependent rates, the attachment and detachment rates depend continuously on the number of attached motors. Third, the kinesin motor model requires that a total of K motors are always attached. For example, if there are N up motors attached to the vesicle, then there must be \(M=K-N\) down motors attached. Importantly, we derive the motor detachment rates and assume instantaneous reattachment of either species. We now proceed with the derivation.
In the symmetric kinesin model, the forces exerted in the up and down directions are given by
where \(F_s\) is the motor stall force, U is the cargo velocity, and \(V_m\) is the free-moving velocity of a single motor. Instantaneous force–balance requires that
where N and M are the number of attached motors pushing in the up and down directions, respectively. Note that this equation is the same starting point considered by Allard et al. (2019), but we have included a drag force \(\zeta U\). Moreover, this equation is the discrete analogue of our force–balance equation, \(\phi F_{-A}(U) + (1-\phi ) F_A(U) -\zeta U = 0\). In contrast to our formulation, it is possible to solve explicitly for the velocity. Solving for U yields,
Plugging into Eq. (29) allows us to write the up and down forces in terms of the total motors attached:
Attachment and detachment rates follow from Bell’s law (Bell 1978):
Plugging Eq. (31) into Eq. (32), the attachment and detachment rates become
where we take \(M=K-N\). To non-dimensionalize, we take \(\gamma = 2F_s/f_0\), \({{\tilde{\zeta }}} = \zeta V_m/(2 F_s)\), and \(\kappa _0 = {\bar{\kappa }}_0/2\). The non-dimensional parameters yield,
where \(\gamma = 2F_s/f_0\), \({\tilde{\zeta }} = \zeta V_m/(2 F_s)\), and \(\kappa _0 = {\bar{\kappa }}_0/2\). The \(\gamma \) and \(\kappa _0\) parameters are identical to the parameters used in Allard. The \({\tilde{\zeta }}\) term is the non-dimensionalized drag. We abuse notation and define \(\zeta \equiv {\tilde{\zeta }}\). The continuum limit version of these equations is taken by noting that
i.e., viscous drag modifies the continuum variable \(x:=M/K\) by changing the slope and y-intercept of the input to the exponential functions. Thus, the continuum approximation is given by
where \(a=1/(1+2\zeta /K)\), \(b=\zeta /(K+2\zeta )\). Using standard methods (Gardiner 2009), the deterministic equation is
Figure 9b shows the phase line of the deterministic dynamics for different values of \(\zeta \). Note that stable motor states inform us directly of stable velocities using Eq. (30). The magnitude and sign of the velocity are determined by the difference in the number of attached and unattached motors. For example, if the motor states \(x=0.1\) and \(x=0.9\) are stable, these points correspond to distinct negative and positive velocities. Therefore, we use Fig. 9b as a proxy for determining the existence of multistable velocities in the symmetric kinesin model.
A comparison of bifurcations in the lubrication and symmetric kinesin models. a Example force–velocity curves from the lubrication model with different values of viscous drag. Here, we use the parameters \(\pi _1=1\), \(\pi _3=1\), \(\pi _4=4.7\), \(\pi _5=0.1\), \(\phi =0.5\). b Corresponding one-parameter bifurcation. Multistability terminates through saddle-node (SN) bifurcations c Phase line curves in the symmetric kinesin model. d One-parameter bifurcation diagram of the symmetric kinesin model in \(\zeta \). For moderate values of viscous drag \(\zeta \), there are two stable motor states. As the drag increases, the system undergoes a pitchfork bifurcation. Beyond the pitchfork, only equal numbers of motors from both species are attached, and therefore, only the zero velocity is stable. The parameters used in the kinesin motor model are \(\kappa _0=0.5\), \(\gamma =3\), \(K=35\) (Color figure online)
We include the lubrication force–velocity curves in Fig. 9a and its corresponding bifurcation diagram in Panel B as a reminder of how multistability changes as a function of drag. For this comparison, we can only take \(\phi =0.5\). Recall that in the lubrication model, nontrivial velocities exist for a range of \(\zeta \) up to the saddle-node (SN) bifurcation. The stable nonzero velocities terminate through a pair of saddle-node bifurcations and leave one stable zero velocity.
Figure 9c shows a similar a one-parameter diagram of the deterministic symmetric kinesin model. For a range of \(\zeta \) up to the pitchfork bifurcation, two different motor states and therefore two different velocities are stable. Beyond the pitchfork, only one stable zero-velocity solution persists.
In summary, the specifics of the lubrication and symmetric kinesin models differ substantially. The symmetric kinesin models do not take into account motor head positions and have attachment and detachment rates dependent on the number of different species attached. In contrast, the myosin model includes position-dependent forces, and it is this position dependence that gives rise to local extrema near zero velocity in the myosin force–velocity curves. Thus, the two models lose multistability through different bifurcations. However, the broad qualitative behaviors are similar: Multistable cargo velocities exist for moderate values of \(\zeta \), and sufficiently large drag values cause the cargo to stop moving.
Rights and permissions
About this article
Cite this article
Park, Y., Fai, T.G. Dynamics of Vesicles Driven Into Closed Constrictions by Molecular Motors. Bull Math Biol 82, 141 (2020). https://doi.org/10.1007/s11538-020-00820-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00820-0