Abstract
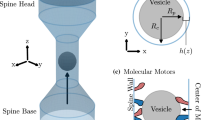
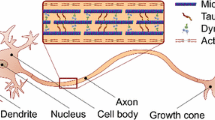
In this study, we consider axonal transport of large cargo vesicles characterised by transient expansion of the axon shaft. Our goal is to formulate a mathematical model which captures the dynamic mechanical interaction of such cargo vesicles with the membrane associated periodic cytoskeletal structure (MPS). It consists of regularly spaced actin rings that are transversal to the longitudinal direction of the axon and involved in the radial contraction of the axon. A system of force balance equations is formulated by which we describe the transversal rings as visco-elastic Kelvin-Voigt elements. In a homogenisation limit, we reformulate the model as a free boundary problem for the interaction of the submembranous MPS with the large vesicle. We derive a non-linear force-velocity relation as a quasi-steady state solution. Computationally we analyse the vesicle size dependence of the transport speed and use an asymptotic approximation to formulate it as a power law that can be tested experimentally.











Similar content being viewed by others
References
Altick AL, Baryshnikova LM, Vu TQ, von Bartheld CS (2009) engQuantitative analysis of multivesicular bodies (mvbs) in the hypoglossal nerve: evidence that neurotrophic factors do not use mvbs for retrograde axonal transport. engJ Compar Neurol 514(6):641–657
Bar-Ziv R, Tlusty T, Moses E, Safran S, Bershadsky A (1999) Pearling in cells: a clue to understanding cell shape. Proc Natl Acad Sci USA 96(18):10140–10145
Chowdary PD, Che DL, Zhang K, Cui B (2015) engRetrograde ngf axonal transport-motor coordination in the unidirectional motility regime. engBiophys J 108(11):2691–2703
Danielli D (2020) engAn overview of the obstacle problem. engNot Am Math Soc 67(10):1487–1497
Datar A, Ameeramja J, Bhat A, Srivastava R, Mishra A, Bernal R, Prost J, Callan-Jones A, Pullarkat P (2019) The roles of microtubules and membrane tension in axonal beading, retraction, and atrophy. Biophys J 117(5):880–891
Fan A, Tofangchi A, Kandel M, Popescu G, Saif T (2017) Coupled circumferential and axial tension driven by actin and myosin influences in vivo axon diameter. Sci Rep 7(1):14188
Fisher ME, Kolomeisky AB (1999) engThe force exerted by a molecular motor. engProc Natl Acad Sci PNAS 96(12):6597–6602
Guedes-Dias P, Holzbaur ELF (2019) EngAxonal transport: driving synaptic function. engSci (Am Assoc Adv Sci) 366(6462):199
Hammarlund M, Jorgensen E, Bastiani M (2007) Axons break in animals lacking \(\beta \)-spectrin. J Cell Biol 176(3):269–275
Han B, Zhou R, Xia C, Zhuang X (2017) Structural organization of the actin-spectrin-based membrane skeleton in dendrites and soma of neurons. Proc Natl Acad Sci USA 114(32):E6678–E6685
Kunwar A, Tripathy SK, Xu J, Mattson MK, Anand P, Sigua R, Vershinin M, McKenney RJ, Yu CC, Mogilner A, Gross SP (2011) engMechanical stochastic tug-of-war models cannot explain bidirectional lipid-droplet transport. engProc Natl Acad Sci PNAS 108(47):18960–18965
Leite S, Sampaio P, Sousa V, Nogueira-Rodrigues J, Pinto-Costa R, Peters L, Brites P, Sousa M (2016) The actin-binding protein \(\alpha \)-adducin is required for maintaining axon diameter. Cell Rep 15(3):490–498
Liewald D, Miller R, Logothetis N, Wagner H-J, Schüz A (2014) Distribution of axon diameters in cortical white matter: an electron-microscopic study on three human brains and a macaque. Biol Cybern 108(5):541–557
McBride HM, Neuspiel M, Wasiak S (2006) engMitochondria: more than just a powerhouse. engCurr Biol 16(14):R551–R560
Mizushima N, Ohsumi Y, Yoshimori T (2002) engAutophagosome formation in mammalian cells. engCell Struct Funct 27(6):421–429
Oelz DB, Rubinstein BY, Mogilner A (2015) engA combination of actin treadmilling and cross-linking drives contraction of random actomyosin arrays. engBiophys J 109(9):1818–1829
Oelz DB, del Castillo U, Gelfand VI, Mogilner A (2018) engMicrotubule dynamics, kinesin-1 sliding, and dynein action drive growth of cell processes. engBiophys J 115(8):1614–1624
Pan X, Zhou Y, Hotulainen P, Meunier F, Wang T (2021) The axonal radial contractility: Structural basis underlying a new form of neural plasticity. BioEssays 43(8) cited By 0
Prokop A (2020) Cytoskeletal organization of axons in vertebrates and invertebrates. J Cell Biol 219(7):05
Roy S (2014) engSeeing the unseen: the hidden world of slow axonal transport. engThe Neurosci (Baltimore, Md.) 20(1):71–81
Tam AK, Mogilner A, Oelz DB (2021) engProtein friction and filament bending facilitate contraction of disordered actomyosin networks. engBiophys J 120(18):4029–4040
Unsain N, Stefani FD, Cáceres A (2018) The actin/spectrin membrane-associated periodic skeleton in neurons. Front Synap Neurosci 10
Vassilopoulos S, Gibaud S, Jimenez A, Caillol G, Leterrier C (2019) Ultrastructure of the axonal periodic scaffold reveals a braid-like organization of actin rings. Nat Commun 10(1) cited By 41
Wang T, Li W, Martin S, Papadopulos A, Joensuu M, Liu C, Jiang A, Shamsollahi G, Amor R, Lanoue V, Padmanabhan P, Meunier FA (2020) engRadial contractility of actomyosin rings facilitates axonal trafficking and structural stability. engThe J Cell Biol 219:5
Wortman J, Shrestha U, Barry D, Garcia M, Gross S, Yu C (2014) Axonal transport: how high microtubule density can compensate for boundary effects in small-caliber axons. Biophys J 106(4):813–823
Xu K, Zhong G, Zhuang X (2013) engActin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. engSci (Am Assoc Advancem Sci) 339(6118):452–456
Acknowledgements
The authors acknowledge funding from the Australian Research Council (ARC) Discovery Program (Grant No. DP180102956), awarded to D. B. O. An RTP scholarship funded by the University of Queensland (UQ), awarded to N. R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
A: Asymptotic expansion of transport velocity
Starting with the first equation in (22), we have the following integral equation
Now we substitute h and its derivative given in (7) obtaining
Coupling this to the asymptotic expansions of A and B given in (29) and (27) we conclude that
where we used \(R_v=1+\varepsilon ^2\). Finally, substitute the asymptotic expansion for V into (32),
Equating coefficients of equal powers of \(\varepsilon \) we obtain
B: Numerical test of quasi-steady state approximation
In this supplementary section we numerically test the validity of the quasi-steady state approximation (23). To this end we solve the time discrete model (8)–(9) numerically, extract the cargo velocity and compare it to the result obtained from numerically solving (23). The numerical results coincide (Fig. 12).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahman, N., Oelz, D.B. A mathematical model for axonal transport of large cargo vesicles. J. Math. Biol. 88, 1 (2024). https://doi.org/10.1007/s00285-023-02022-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-023-02022-3