Abstract
Social predators benefit from cooperation in the form of increased hunting success, but may be at higher risk of disease infection due to living in groups. Here, we use mathematical modeling to investigate the impact of disease transmission on the population dynamics benefits provided by group hunting. We consider a predator–prey model with foraging facilitation that can induce strong Allee effects in the predators. We extend this model by an infectious disease spreading horizontally and vertically in the predator population. The model is a system of three nonlinear differential equations. We analyze the equilibrium points and their stability as well as one- and two-parameter bifurcations. Our results show that weakly cooperating predators go unconditionally extinct for highly transmissible diseases. By contrast, if cooperation is strong enough, the social behavior mediates conditional predator persistence. The system is bistable, such that small predator populations are driven extinct by the disease or a lack of prey, and large predator populations survive because of their cooperation even though they would be doomed to extinction in the absence of group hunting. We identify a critical cooperation level that is needed to avoid the possibility of unconditional predator extinction. We also investigate how transmissibility and cooperation affect the stability of predator–prey dynamics. The introduction of parasites may be fatal for small populations of social predators that decline for other reasons. For invasive predators that cooperate strongly, biocontrol by releasing parasites alone may not be sufficient.






Similar content being viewed by others
Change history
29 August 2018
In the original article, the second author’s family name was misspelled. The correct name is Marta Paliaga.
References
Allee WC (1931) Animal aggregations: a study in general sociology. University of Chicago Press, Chicago
Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, Dobson AP, Ezenwa V, Jones KE, Pedersen AB, Poss M, Pulliam JRC (2003) Social organization and parasite risk in mammals: integrating theory and empirical studies. Ann Rev Ecol Evol Syst 34:517–547
Anderson RM (1982) Theoretical basis for the use of pathogens as biological control agents of pest species. Parasitology 84:3–33
Bate AM, Hilker FM (2013) Complex dynamics in an eco-epidemiological model. Bull Math Biol 75:2059–2078
Bate AM, Hilker FM (2014) Disease in group-defending prey can benefit predators. Theor Ecol 7:87–100
Beauchamp G (2014) Social predation: how group living benefits predators and prey. Academic Press, London
Berec L (2010) Impacts of foraging facilitation among predators on predator–prey dynamics. Bull Math Biol 72:94–121
Berec L, Maxin D (2013) Fatal or harmless: extreme bistability induced by sterilizing, sexually transmitted pathogens. Bull Math Biol 75:258–273
Berec L, Boukal DS, Berec M (2001) Linking the Allee effect, sexual reproduction, and temperature-dependent sex determination via spatial dynamics. Am Nat 157:217–230
Bester MN, Bloomer JP, van Aarde RJ, Erasmus BH, van Rensburg PJJ, Skinner JD, Howell PG, Naude TW (2002) A review of the successful eradication of feral cats from sub-Antarctic Marion Island, Southern Indian Ocean. S Afr J Wildl Res 32:65–73
Busenberg S, van den Driessche P (1990) Analysis of a disease transmission model in a population with varying size. J Math Biol 28:257–270
Côté IM, Poulin R (1995) Parasitism and group size in social animals: a meta-analysis. Behav Ecol 6:159–165
Courchamp F, Macdonald DW (2001) Crucial importance of pack size in the African wild dog Lycaon pictus. Anim Conserv 4:169–174
Courchamp F, Sugihara G (1999) Modeling the biological control of an alien predator to protect island species from extinction. Ecol Appl 9:112–123
Courchamp F, Berec L, Gascoigne J (2008) Allee effects in ecology and conservation. Oxford University Press, New York
Francomano E, Hilker FM, Paliaga M, Venturino E (2016) On basins of attraction for a predator-prey model via meshless approximation. AIP Conference Proceedings 1776(1):070007. doi:10.1063/1.4965353
Francomano E, Hilker FM, Paliaga M, Venturino E (2017) An efficient method to reconstruct invariant manifolds of saddle points. Dolomit Res Notes Approx 10:25–30
Francomano E, Hilker FM, Paliaga M, Venturino E (in press) Separatrix reconstruction to identify tipping points in an eco-epidemiological model. Appl Math Comput. doi:10.1016/j.amc.2017.07.022
Hilker FM (2009) Epidemiological models with demographic Allee effect. In: Mondaini RP (ed). In: Biomat 2008: international symposium on mathematical and computational biology. World Scientific, Singapore, pp 52–77
Hilker FM (2010) Population collapse to extinction: the catastrophic combination of parasitism and Allee effect. J Biol Dyn 4:86–101
Hilker FM, Schmitz K (2008) Disease-induced stabilization of predator–prey oscillations. J Theor Biol 255:299–306
Hilker FM, Langlais M, Malchow H (2009) The Allee effect and infectious diseases: extinction, multistability, and the (dis-)appearance of oscillations. Am Nat 173:72–88
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Loehle C (1995) Social barriers to pathogen transmission in wild animal populations. Ecology 76:326–335
Mangin S, Gauthier-Clerc M, Frenot Y, Gendner JP, Le Maho Y (2003) Ticks Ixodes uriae and the breeding performance of a colonial seabird, king penguin Aptenodytes patagonicus. J Avian Biol 34:30–34
McMahon TA, Finlayson BL, Haines AT, Srikanthan R (1992) Global runoff: continental comparisons of annual flows and peak discharges. Catena Verlag, Cremlingen
Mena-Lorca J, Hethcote HW (1992) Dynamic models of infectious diseases as regulator of population sizes. J Math Biol 30:693–716
Numfor E, Hilker FM, Lenhart S (2017) Optimal culling and biocontrol in a predator–prey model. Bull Math Biol 79:88–116
Oliveira NM, Hilker FM (2010) Modelling disease introduction as biological control of invasive predators to preserve endangered prey. Bull Math Biol 72:444–468
Packer C, Ruttan L (1988) The evolution of cooperative hunting. Am Nat 132:159–198
Rubenstein DI (1978) On predation, competition, and the advantages of group living. In: Bateson PPG, Klopfer PH (eds) Social behavior. Plenum Press, New York, pp 205–231
Rubenstein DR, Lovette IJ (2007) Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr Biol 17:1414–1419
Teixeira Alves M, Hilker FM (2017) Hunting cooperation and Allee effects in predators. J Theor Biol 419:13–22
Thieme HR, Dhirasakdanon T, Han Z, Trevino R (2009) Species decline and extinction: synergy of infectious diseases and Allee effect? J Biol Dyn 3:305–323
Vander Zanden MJ, Hansen GJA, Higgins SN, Kornis MS (2010) A pound of prevention, plus a pound of cure: early detection and eradication of invasive species in the Laurentian Great Lakes. J Gt Lakes Res 36:199–205
Venturino E (2016) Ecoepidemiology: a more comprehensive view of population interactions. Math Modell Nat Phenom 11:49–90
Ward P, Enders M (1985) Conflict and cooperation in the group feeding of the social spider Stegodyphus mimosarum. Behavior 94:167–182
Watts DP, Mitani JC (2002) Hunting behavior of chimpanzees at Ngogo, Kibale National Park, Uganda. Int J Primatol 23:1–28
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Isocline Analysis in the Endemic Predator–Prey Phase Plane
We perform an isocline analysis of the three-dimensional model (5)–(7), which will lead us to a reduced model in the two-dimensional phase plane. Any nontrivial equilibrium satisfies the following zero-growth conditions
Substituting the value of n from equation (11) into equation (12), we find the nontrivial prevalence value at any equilibrium with \(p^*>0\)
which is exactly expression (9) shown in the main text.
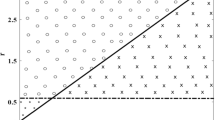
We can now intersect the three-dimensional state space with the prevalence nullplane \(i=i^*\) and work on the (p, n) plane, where \(p>0\) and \(n>0\). This simplifies the analysis because it allows us to find the intersection of curves (10) and (11) with \(i=i^*\) constant (see Fig. 1).
The parabola (10) intersects the n–axis at the point (0, k), while the hyperbola (11) intersects the n–axis at the point \((0, 1+ \mu i^*)\). That is, if the vertical intercept of the parabola (solid red dashed curve in Fig. 1) is higher than the vertical intercept of the hyperbola (dashed blue curve in Fig. 1), then there is a unique intersection of the parabola and hyperbola (Fig. 1b, d). This condition can be expressed as \(k>1+\mu i^*\) or equivalently as \({\mathcal R}_p(i^*)>1\). If \({\mathcal R}_p(i^*)<1\), there can be 0, 1, or 2 intersections (Fig. 1a, c).
Appendix B: Existence and Stability of the Stationary States
To analyze the equilibrium point, we consider the Jacobian of system (5)–(7):
where \(b'= \beta -\mu \), \(\theta ' = 1-\theta \), \(\alpha ' =1+\alpha p\), and \(p'=1+2\alpha p\). The system has the following possible equilibria:
-
1.
\(E_{0} = (0,0,0)\). The trivial extinction state always exists and is always unstable, since the eigenvalues of the Jacobian evaluated at this equilibrium are
$$\begin{aligned} \lambda _{1} = r > 0 , \quad \lambda _{2}= -1+k, \quad \lambda _{3}= \beta - \mu -(1 -\theta ) k. \end{aligned}$$ -
2.
\(E_\mathrm{n} = (k,0,0)\). This represents the disease- and predator-free equilibrium with the prey being at carrying capacity. It always exists and its eigenvalues are
$$\begin{aligned} \lambda _{1}=-r < 0,\quad \lambda _{2}= -1+k, \quad \lambda _{3}= \beta - \mu -(1 -\theta ) k. \end{aligned}$$Hence, \(E_\mathrm{n}\) is stable if
$$\begin{aligned} k<1, \quad {\mathcal R}_i=\frac{\beta +\theta k}{\mu +k}<1. \end{aligned}$$ -
3.
\(E_{i}= (0,0,1)\). This is the disease-induced extinction state with both predators and prey being absent. It always exists, but it is always unstable because its eigenvalues are:
$$\begin{aligned} \lambda _{1}=r > 0,\quad \lambda _{2}= -1+\mu ,\quad \lambda _{3}= \beta - \mu -(1 -\theta ) k. \end{aligned}$$ -
4.
\(E_\mathrm{ni}= (k,0,i^{\dagger })\), where \(i^{\dagger } = 1-\frac{(1-\theta )k}{\beta -\mu }\). This is the state corresponding to disease-induced predator extinction, with the prey reaching carrying capacity. It exists if
$$\begin{aligned} {\mathcal R}_i=\frac{\beta +\theta k}{\mu +k}>1. \end{aligned}$$The eigenvalues are
$$\begin{aligned} \lambda _{1}=-r,\quad \lambda _{2}= k-(1+ \mu i^{\dagger }), \quad \lambda _{3}= (\beta - \mu )(1- 2i^{\dagger }) -(1 -\theta ) k. \end{aligned}$$That is, \(E_\mathrm{ni}\) is stable if \(k<1+\mu i^{\dagger }\), which translates into \({\mathcal R}_p(i^*)<1\).
-
5.
\(E_\mathrm{np}= (n^{\circ },p^{\circ },0)\) is the disease-free coexistence state of predators and prey. The values of \(n^{\circ }\) and \(p^{\circ }\) are cumbersome to obtain. If \(k>1\), \(E_\mathrm{np}\) is unique. If \(k<1\), there can be up to two equilibria \(E_\mathrm{np}\) which (dis-)appear in a saddle–node bifurcation in the disease-free plane (see Teixeira Alves and Hilker 2017). The stability of \(E_\mathrm{np}\) is investigated numerically and discussed in the main text.
-
6.
\(E_\mathrm{npi}= (n^*,p^*,i^*)\), with \(i^*= [\beta -\mu -(1-\theta )](\beta -\mu \theta )^{-1}\) as shown in Appendix A. This equilibrium is the endemic coexistence state where all three species (prey, predators and disease) coexist. As for the disease-free coexistence equilibrium, we cannot find the explicit values of the prey and predator values. From \(i^*>0\), we obtain that a necessary existence condition for \(E_\mathrm{npi}\) is
$$\begin{aligned} {\mathcal R}_0(0,1)=\frac{\beta +\theta }{1+\mu }>1. \end{aligned}$$In Sect. 3, we find that if \({\mathcal R}_p(i^*)>1\) the equilibrium exists and is unique and stable. If \({\mathcal R}_p(i^*)<1\), there can be two, one or no equilibrium point \(E_\mathrm{npi}\). We study the existence and stability of \(E_\mathrm{npi}\) numerically in the main text and show that there is a saddle–node bifurcation when varying \(\alpha \).
Rights and permissions
About this article
Cite this article
Hilker, F.M., Paliga, M. & Venturino, E. Diseased Social Predators. Bull Math Biol 79, 2175–2196 (2017). https://doi.org/10.1007/s11538-017-0325-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0325-y