Abstract
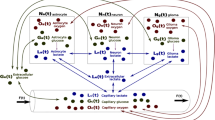
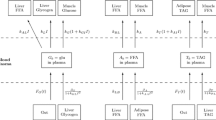
The mammalian kidney consumes a large amount of energy to support the reabsorptive work it needs to excrete metabolic wastes and to maintain homeostasis. Part of that energy is supplied via the metabolism of glucose. To gain insights into the transport and metabolic processes in the kidney, we have developed a detailed model of the renal medulla of the rat kidney. The model represents water and solute flows, transmural fluxes, and biochemical reactions in the luminal fluid of the nephrons and vessels. In particular, the model simulates the metabolism of oxygen and glucose. Using that model, we have identified parameters concerning glucose transport and basal metabolism that yield predicted blood glucose concentrations that are consistent with experimental measurements. The model predicts substantial axial gradients in blood glucose levels along various medullary structures. Furthermore, the model predicts that in the inner medulla, owing to the relatively limited blood flow and low tissue oxygen tension, anaerobic metabolism of glucose dominates.








Similar content being viewed by others
References
Bagnasco S, Good D, Balaban R, Burg M (1985) Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Physiol 248:F522–F526
Chen J, Edwards A, Layton AT (2009a) A mathematical model of oxygen transport in the rat outer medulla: II. Impacts of outer medullary architecture. Am J Physiol Renal Physiol 297:F537–F548
Chen J, Edwards A, Layton AT (2009b) Effects of pH and medullary blood flow on oxygen transport and sodium reabsorption in the rat outer medulla. Am J Physiol Renal Physiol 298(6):F1369–F1383
Chen J, Layton AT, Edwards A (2009c) A mathematical model of oxygen transport in the rat outer medulla: I. Model formulation and baseline results. Am J Physiol Renal Physiol 297:F517–F536
Dickman KG, Mandel LJ (1990) Differential effects of respiratory inhibitors on glycolysis in proximal tubules. Am J Physiol 258:F1608–F1615
Edwards A, Layton AT (2011) Modulation of outer medullary NaCl transport and oxygenation by nitric oxide and superoxide. Am J Physiol Renal Physiol 301:F979–F996
Fine LG, Norman JT (2008) Chronic hypoxia as a mechanism of progression of chronic kidney disease: form hypothesis to novel therapeutics. Kidney Int 74:867–872
Fine LG, Bandyopadhay D, Norman JT (2000) Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl 75:S22–S26
Foley RN, Collins AJ (2007) End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18:2644–2648
Friederick-Persson M, Thorn E, Hansell P, Nangaku M, Palm F (2013) Kidney hypoxia, attributable to increased oxygen consumption, induces nephropathy independently of hyperglycemia and oxidative stress. Hypertension 62:914–919
Fry BC, Edwards A, Sgouralis I, Layton AT (2014) Impact of renal medullary three-dimensional architecture on oxygen transport. Am J Physiol Renal Physiol 307:F263–F272
Fry BC, Edwards A, Layton AT (2015) Impacts of nitric oxide and superoxide on renal medullary oxygen transport and urine concentration. Am J Physiol Renal Physiol 308:F967–F980
Fry BC, Edwards A, Layton AT (2016) Impact of nitric-oxide-mediated vasodilation and oxidative stress on renal medullary oxygenation: a modeling study. Am J Physiol Renal Physiol 310:F237–F247
Ganguli M, Tobian L (1974) Does the kidney autoregulate papillary plasma flow in chronic postsalt hypertension? Am J Physiol 226:330–333
Greger R, Schlatter E, Lang F (1983) Evidence for electroneutral sodium chloride cotransport in the cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflügers Arch 396:308–314
Hervy S, Thomas SR (2003) Inner medullary lactate production and urine-concentrating mechanism: a flat medullary model. Am J Physiol Renal Physiol 284:F65–F81
Jen JF, Stephenson JL (1994) Externally driven countercurrent multiplication in a mathematical model of the urinary concentrating mechanism of the renal inner medulla. Bull Math Biol 56:491–514
Klein Keith I, Wang Maw-Song, Torikai Shozo, Davidson Warren, Kurokawa Kiyoshi (1980) Substrate oxidation by defined single nephron segments of rat kidney. Int J Biochem 12(1):53–54
Kramer K, Thurau K, Deetjen P (1960) Hämodynamik des nierenmarks. Pflügers Arch Eur J Physiol 270(3):251–269
Kriz W (1967) Der architektonische and funktionelle Aufbau der Rattenniere. Z Zellforsch 82:495–535
Kriz W (1981) Structural organization of the renal medulla: comparative and functional aspects. Am J Physiol (Regul Integr Comp Physiol 10) 241:R3–R16
Kriz W, Schnermann J, Koepsell H (1972) The position of short and long loops of Henle in the rat kidney. Z Anat Entwickl-Gesch 138:301–319
Layton AT (2011a) A mathematical model of the urine concentrating mechanism in the rat renal medulla: I. Formulation and base-case results. Am J Physiol Renal Physiol 300:F356–F371
Layton AT (2011b) A mathematical model of the urine concentrating mechanism in the rat renal medulla: II. Functional implications of three-dimensional architecture. Am J Physiol Renal Physiol 300:F372–F394
Layton AT, Dantzler WH, Pannabecker TL (2012) Urine concentrating mechanism: Impact of vascular and tubular architecture and a proposed descending limb urea-Na\(^+\) cotransporter. Am J Physiol Renal Physiol 302:F591–F605
Layton AT, Layton HE (2005) A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla: I. Formulation and base-case results. Am J Physiol Renal Physiol 289:F1346–F1366
Nelimarkka O (1984) Renal oxygen and lactate metabolism in hemorrhagic shock. An experimental study. Acta Chir Scand Suppl 518:1–44
Nieves-Gonzalez A, Clausen C, Layton AT, Layton HE, Moore LC (2013) Transport efficiency and workload distribution in a mathematical model of the thick ascending limb. Am J Physiol Renal Physiol 304:F653–F664
Pannabecker TL, Abbott DE, Dantzler WH (2004) Three-dimensional functional reconstruction of inner medullary thin limbs of Henle’s loop. Am J Physiol Renal Physiol 286:F38–F45
Rich PR (2003) The molecular machinery of Keilin’s respiratory chain. Biochem Soc Trans 31:1095–1106
Ruiz-Guinazu A, Pehling G, Rumrich G, Ullrich KJ (1961) Glucose and lactic acid concentration at the peak of the vascular counterflow system in the renal medulla. Pflügers Arch 274:311–317
Stern MD, Bowen PD, Parma R, Osgood RW, Bowman RL, Stein JH (1979) Measurement of renal cortical and medullary blood flow by laser-Doppler spectroscopy in the rat. Am J Physiol Renal Physiol 236:F80–F87
Stokes JB, Grupp C, Kinne RKH (1987) Purification of rat papillary collecting duct cells: functional and metabolic assessment. Am J Physiol 253:F251–F262
Tanaka S, Tanaka T, Nnagaku M (2014) Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307:F1187–F1195
Thomas SR (2000) Inner medullary lactate production and accumulation: a vasa recta model. Am J Physiol Renal Physiol 279:F468–F481
Thomas SR, Wexler AS (1995) Inner medullary external osmotic driving force in a 3-D model of the renal concentrating mechanism. Am J Physiol (Renal Fluid Electrolyte Physiol 38) 269:F159–F171
Uchida S, Endou H (1988) Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol Renal Physiol 255:F977–F983
Weidemann MJ, Krebs HA (1969) The fuel of respiration of rat kidney cortex. Biochem J 112:149–166
Weinstein AM (1998) A mathematical model of the inner medullary collecting duct of the rat: pathways for Na and K transport. Am J Physiol (Renal Physiol 43) 274:F841–F855
Zeidel ML, Silva P, Seifter JL (1986) Intracellular pH regulation and proton transport by rabbit renal medullary collecting duct cells: role of plasma membrane proton adenosine triphosphatase. J Clin Invest 77:113–120
Zhang W, Edwards A (2006) A model of glucose transport and conversion to lactate in the renal medullary microcirculation. Am J Physiol Renal Physiol 290:F87–F102
Acknowledgments
This work was supported in part by the National Science Foundation through Grant DMS-1263995 and the National Institutes of Health through Grants DK089066 and DK106102 to A. Layton.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Fry, B.C. & Layton, A.T. Modeling Glucose Metabolism in the Kidney. Bull Math Biol 78, 1318–1336 (2016). https://doi.org/10.1007/s11538-016-0188-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-016-0188-7