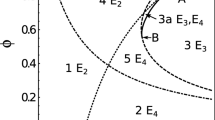
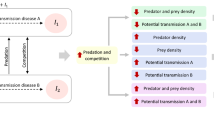
Abstract
The virulent effects of a pathogen on host fecundity and mortality (both intrinsic and extrinsic mortality due to predation) often increase with the age of infection. Age of infection often is also correlated with parasite fitness, in terms of the number of both infective propagules produced and the between-host transmission rate. We introduce a four-population partial differential equations (PDE) model to investigate the invasibility and prevalence of an obligately killing fungal parasite in a zooplankton host as they are embedded in an ecological network of predators and resources. Our results provide key insights into the role of ecological interactions that vary with the age of infection. First, selective predation, which is known both theoretically and empirically to reduce disease prevalence, does not always limit disease spread. This condition dependency relies on the timing and intensity of selective predation and how that interacts with the direct effects of the parasite on host mortality. Second, low host resources and intense predation can prevent disease spread, but once conditions allow the invasion of the parasite, the qualitative dynamics of the system do not depend on the intensity of the selective predation. Third, a comparison of the PDE model with a model based on ordinary differential equations (ODE model) reveals a parametrization for the ODE version that yields an endemic steady state and basic reproductive ratio that are identical to those in the PDE model. Our results highlight the complexity of resource–host–parasite–predator interactions and suggest the need for additional data–theory coupling exploring how community ecology influences the spread of infectious diseases.









Similar content being viewed by others
References
Alizon S, Hurford A, Mideo N, van Baalen M (2009) Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol 22:245–259
Anderson RM, May RM (1991) Infectious diseases of humans. Oxford Science Publications, Oxford
Auld SKJR, Hall SR, Duffy MA (2012) Epidemiology of a Daphnia-multiparasite system and its implications for the Red Queen. PLoS One 7:e39564
Auld SKJR, Hall SR, Ochs JH, Sebastian M, Duffy MA (2014) Predators and patterns of within-host growth can mediate both among-host competition and the evolution of transmission potential of parasites. Am Nat 184:S77–S90
Bedhomme S, Agnew P, Sidobre C, Michalakis Y (2004) Virulence reaction norms across a food gradient. Proc R Soc Lond B Biol Sci 271:739–744
Bertram CR, Pinkowski M, Hall SR, Duffy MA, Cáceres CE (2013) Trait-mediated indirect effects, predators, and disease: test of a size-based model. Oecologia 173:1023–1032
Bittner K, Rothhaupt K-O, Ebert D (2002) Ecological Interactions of the microparasite Caullerya mesnili and its host Daphnia galeata. Limnol Oceanogr 47:300–305
Boots M, Mealor M (2007) Local interactions select for lower pathogen infectivity. Science 315:1284–1286
Bull JJ (1994) Virulence. Evolution 48:1423–1437
Cáceres CE, Hall SR, Duffy MA, Tessier AJ, Helmle C, MacIntyre S (2006) Physical structure of lakes constrains epidemics in Daphnia populations. Ecology 87:1438–1444
Cáceres CE, Knight CJ, Hall SR (2009) Predators-spreaders: predation can enhance parasite success in a planktonic host–parasite system. Ecology 90:2850–2858
Cáceres CE, Davis G, Duple S, Hall SR, Koss A, Lee P, Rapti Z (2014) Complex Daphnia interactions with parasites and competitors. Math Biosci 258:148–161
Carius HJ, Little TJ, Ebert D (2001) Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution 55:1136–1145
Choisy M, Rohani P (2006) Harvesting can increase severity of wildlife disease epidemics. Proc R Soc Lond B Biol Sci 273:2015–2034
Choo K, Williams PD, Day T (2003) Host mortality, predation and the evolution of parasite virulence. Ecol Lett 6:310–315
Day T (2001) Parasite transmission modes and the evolution of virulence. Evolution 55:2389–2400
Day T (2002) Virulence evolution via host exploitation and toxin production in spore-producing pathogens. Ecol Lett 5:471–476
Day T (2003) Virulence evolution and the timing of disease life-history events. Trends Ecol Evol 18:113–118
Day T, Alizon S, Mideo N (2011) Bridging scales in the evolution of infectious disease life histories: theory. Evolution 65:3448–3461
Duffy MA, Hall SR (2008) Selective predation and rapid evolution can jointly dampen effects of virulent parasites on Daphnia populations. Am Nat 171:499–510
Duffy MA (2009) Staying alive: the post-consumption fate of parasite spores and its implications for disease dynamics. Limnol Oceanogr 54:770–773
Duffy MA, Cáceres CE, Hall SR, Tessier AJ, Ives AR (2010) Temporal, spatial and between-host comparisons of patterns of parasitism in lake zooplankton. Ecology 91:3322–3331
Dwyer G, Dushoff J, Yee SH (2004) The combined effects of pathogens and predators on insect outbreaks. Nature 430:341–345
Ebert D (2005) Ecology, epidemiology, and evolution of parasitism in Daphnia. National Library of Medicine (US), National Center for Biotechnology Information, Bethesda. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books
Ebert D, Bull JJ (2003) Challenging the trade-off model for the evolution of virulence: is virulence management feasible? Trends Microbiol 11:15–20
Ebert D, Weisser WW (1997) Optimal killing for obligate killers: the evolution of life-histories and virulence of semelparous parasites. Proc R Soc Lond B Biol Sci 264:985–991
Feng Z, Velasco-Hernandez J, Tapia-Santos B (2013) A mathematical model for coupling within-host and between-host dynamics in an environmentally-driven infectious disease. Math Biosci 241:49–55
Gilchrist MA, Sulsky DL, Pringle A (2006) Identifying fitness and optimal life-history strategies for an asexual filamentous fungus. Evolution 60:970–979
Hall SR, Duffy MA, Cáceres CE (2005) Selective predation and productivity jointly drive complex behavior in host–parasite systems. Am Nat 165:70–81
Hall SR, Simonis JL, Nisbet RM, Tessier AJ, Cáceres CE (2009a) Resource ecology of virulence in a planktonic host–parasite system: an explanation using dynamic energy budgets. Am Nat 174:149–162
Hall SR, Knight CJ, Becker CR, Duffy MA, Tessier AJ, Cáceres CE (2009b) Quality matters: resource quality for hosts and the timing of epidemics. Ecol Lett 12:118–128
Hall SR, Becker CR, Duffy MA, Cáceres CE (2010) Variation in resource acquisition and use among hosts can create key epidemiological tradeoffs. Am Nat 176:557–565
Hall SR, Becker CR, Duffy MA, Cáceres CE (2012) A power-efficiency tradeoff in resource use alters epidemiological relationships. Ecology 93:645–656
Hawlena D, Abramsky Z, Bouskila A (2010) Bird predation alters infestation of desert lizards by parasitic mites. Oikos 119:730–736
Hethcote HW (2000) The mathematics of infectious diseases. SIAM Rev 42:599–653
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
Holt RD, Roy M (2007) Predation can increase the prevalence of infectious disease. Am Nat 169:690–699
Hurtado P, Hall SR, Ellner SP (2014) Infectious disease in consumer populations: dynamic consequences of prey-mediated transmission and infectiousness. Theor Ecol 7:163–179
Johnson PTJ, Longcore JE, Stanton DE, Carnegie RB, Shields JD, Preu ER (2006a) Chytrid fungal infections of Daphnia pulicaria development, ecology, pathology and phylogeny of polycaryum laeve. Freshw Biol 51:634–648
Johnson PT, Stanton DE, Preu ER, Forshay KJ, Carpenter SR (2006b) Dining on disease: how interactions between infection and environment affect predation risk. Ecology 87:1973–1980
Lenski RE, May RM (1994) The evolution of virulence in parasites and pathogens: reconciliation between two competing hypotheses. J Theor Biol 169:253–265
Martcheva M, Thieme H (2003) Progression age and enhanced backward bifurcation in an epidemic model with super infection. J Math Biol 46:385–424
May RM, Anderson RM (1983) Parasite–host coevolution. In: Futuyma DJ, Slatkin M (eds) Coevolution. Sinauer, Sunderland, pp 186–206
Mideo N, Nelson WA, Reece SE, Bell AS, Read AF, Day T (2011) Bridging scales in the evolution of infectious disease life histories: application. Evolution 65:3298–3310
Morozov AY, Adamson MW (2011) Evolution of virulence driven by predator–prey interaction: possible consequences for population dynamics. J Theor Biol 276:181–191
Ostfeld RS, Holt RD (2004) Are predators good for your health? Evaluating evidence for top–down regulation of zoonotic disease reservoirs. Front Ecol Environ 2:13–20
Otterstatter MC, Thomson JD (2006) Within-host dynamics of an intestinal pathogen of bumble bees. Parasitology 133:749–761
Packer C, Holt RD, Hudson PJ, Lafferty KD, Dobson AP (2003) Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecol Lett 6:797–802
Peacock S, Connors B, Krkosek M, Irvine JS, Lewis MA (2013) Can reduced predation offset negative effects of sea louse parasites on chum salmon? Proc R Soc Lond B Biol Sci 281(1776):20132913
Prior NH, Washington CN, Housley JM, Hall SR, Duffy MA, Cáceres CE (2011) Maternal effects and epidemiological traits in a planktonic host–parasite system. Evolut Ecol Res 13:401–413
Rosenzweig ML (1971) Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science 171:385–387
Rosenzweig ML, MacArthur RH (1963) Graphical representations and conditions of predator–prey interactions. Am Nat 97:209–223
Sasaki A, Iwasa Y (1991) Optimal growth schedule of pathogens within a host: switching between lytic and latent cycles. Theor Popul Biol 39:201–239
Vale PF, Little TJ (2012) Fecundity compensation and tolerance to a sterilizing pathogen in Daphnia. J Evolut Biol 25:1888–1896
Webb GF, D’Agata EMC, Magal P, Ruan S (2005) A model of antibiotic-resistant bacterial epidemics in hospitals. Proc Natl Acad Sci USA 102:13343–133348
Acknowledgments
We gratefully acknowledge support from the Illinois Campus Research Board through Grant 14038 and the National Science Foundation through Grants DUE-1129198, DEB-1120804 and DEB-1354407. We also thank an anonymous referee for his thoughtful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
is the total infected host population and \(J_0\) is the expected life span of an infected host. Then, \(E_e\) is described by the relationships
Here,
is the expected value of within-host pathogen load in the life span of an infected host,
and
is the expected value of the fecundity reduction parameter over the life span of the infected host,
Solving (9) for \(A_e\) yields a cubic polynomial which in turn can be used to find the equilibrium values for the remaining population densities.
Appendix B
If \(\varLambda \) is real in (6), then it follows by a simple differentiation that \(\frac{\mathrm{d} R}{\mathrm{d} \varLambda }<0\). Hence, if \(R_0=R(0)>1\), it follows that the equation \(R(\varLambda ) =1\) has real positive solutions \(\varLambda \). Therefore, \(R_0>1\) implies instability. Similarly, if \(R_0<1\), then the same argument shows that the equation \(R(\varLambda ) =1\) has no real positive solutions. It remains to be shown that it does not have complex solutions with positive real part either. Let \(\varLambda = x + i y\) be complex. Then, if \(x \ge 0\) and if one sets \(\varLambda _0 = \lambda + \frac{f_S(A_{df})}{A_{df}} S_{df}\) and
it follows \(1=|R(\varLambda )| = \left| \frac{\int _{0}^{\infty } g(a) e^{-xa} e^{-iya} \mathrm{d}a}{x+\varLambda _0+ i y} \right| \le \frac{\int _{0}^{\infty } g(a) e^{-xa} \mathrm{d}a}{x+\varLambda _0} =R(x) \le R(0)=R_0\). Hence, if \(R_0<1\), then the disease-free equilibrium is locally asymptotically stable, and if \(R_0>1\), it is unstable. For a more rigorous justification of this result, one needs to study the semigroup properties of the generator (Martcheva and Thieme 2003).
Rights and permissions
About this article
Cite this article
Rapti, Z., Cáceres, C.E. Effects of Intrinsic and Extrinsic Host Mortality on Disease Spread. Bull Math Biol 78, 235–253 (2016). https://doi.org/10.1007/s11538-016-0141-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-016-0141-9