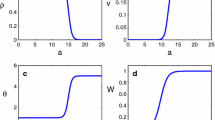
Abstract
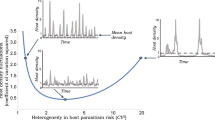
Most evolutionary theory predicts that, during epidemics, hosts will evolve higher resistance to parasites that kill them. Here, we provide an alternative to that typical expectation, with an explanation centered on resource feedbacks. When resistance is costly, hosts evolve decreasing resistance without parasites, as expected. But with parasites, hosts can evolve lower resistance than they would in the absence of parasites. This outcome arises in an eco-evolutionary model when four conditions are met: first, resistance has a fecundity cost (here, via decreased foraging/exposure rate); second, resources increase during epidemics via trophic cascades; third, increased resources magnify the benefit of maintaining a fast foraging rate, thereby magnifying the cost of evolving a slower foraging/exposure rate (i.e., resistance); fourth, that amplification of the cost outweighs the benefit of resistance. When these conditions are met, hosts evolve lower resistance than without parasites. This phenomenon was previously observed in a motivating mesocosm experiment with fungal parasites, zooplankton hosts, and algal resources. Re-analyzing this experiment produced evidence for our model’s mechanism. Thus, both model and experiment indicate that, via resource feedbacks, parasites can counterintuitively select against resistance.







Similar content being viewed by others
References
Agrawal A, Lively CM (2002) Infection genetics: gene-for-gene versus matching-alleles models and all points in between. Evol Ecol Res 4(1):91–107
Altizer S, Harvell D, Friedle E (2003) Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol Evol 18(11):589–596
Antonovics J, Thrall PH (1994) The cost of resistance and the maintenance of genetic polymorphism in host–pathogen systems. Proc R Soc Lond B 257(1349):105–110
Auld SKJR, Penczykowski RM, Ochs JH, Grippi DC, Hall SR, Duffy MA (2013) Variation in costs of parasite resistance among natural host populations. J Evol Biol 26(11):2479–2486. https://doi.org/10.1111/jeb.12243
Auld SKJR, Hall SR, Ochs JH, Sebastian M, Duffy MA (2014) Predators and patterns of within-host growth can mediate both among-host competition and evolution of transmission potential of parasites. Am Nat 184:S77–S90. https://doi.org/10.1086/676927
Best A, White A, Boots M (2017) The evolution of host defence when parasites impact reproduction. Evol Ecol Res 18(4):393–409
Boots M (2011) The evolution of resistance to a parasite is determined by resources. Am Nat 178(2):214–220. https://doi.org/10.1086/660833
Brunner FS, Anaya-Rojas JM, Matthews B, Eizaguirre C (2017) Experimental evidence that parasites drive eco-evolutionary feedbacks. Proc Natl Acad Sci USA 114(14):3678–3683
Buck JC, Ripple WJ (2017) Infectious agents trigger trophic cascades. Trends Ecol Evol 32(9):681–694. https://doi.org/10.1016/j.tree.2017.06.009
Civitello DJ, Penczykowski RM, Hite JL, Duffy MA, Hall SR (2013) Potassium stimulates fungal epidemics in Daphnia by increasing host and parasite reproduction. Ecology 94(2):380–388
Cooper J, Crawford RJ, De Villiers MS, Dyer BM, Hofmeyr GG, Jonker A (2009) Disease outbreaks among penguins at sub-Antarctic Marion Island: a conservation concern. Mar Ornithol 37:193–196
Cortez MH, Ellner SP (2010) Understanding rapid evolution in predator–prey interactions using the theory of fast-slow dynamical systems. Am Nat 176(5):E109–E127
Coulson G, Cripps JK, Garnick S, Bristow V, Beveridge I (2018) Parasite insight: assessing fitness costs, infection risks and foraging benefits relating to gastrointestinal nematodes in wild mammalian herbivores. Philos Trans R Soc B Biol Sci 373(1751):20170197
Daszak P, Cunningham AA, Hyatt AD (2000) Wildlife ecology—emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287(5452):443–449. https://doi.org/10.1126/science.287.5452.443
Duffy MA, Sivars-Becker L (2007) Rapid evolution and ecological host–parasite dynamics. Ecol Lett 10(1):44–53. https://doi.org/10.1111/j.1461-0248.2006.00995.x
Duffy MA, Tessier AJ, Kosnik MA (2004) Testing the ecological relevance of Daphnia species designations. Freshw Biol 49(1):55–64
Duffy MA, Ochs JH, Penczykowski RM, Civitello DJ, Klausmeier CA, Hall SR (2012) Ecological context influences epidemic size and parasite-driven evolution. Science 335(6076):1636–1638. https://doi.org/10.1126/science.1215429
Duncan AB, Fellous S, Kaltz O (2011) Reverse evolution: selection against costly resistance in disease-free microcosm populations of Paramecium caudatum. Evolution 65(12):3462–3474. https://doi.org/10.1111/j.1558-5646.2011.01388.x
Ebert D (2005) Ecology, epidemiology, and evolution of parasitism in Daphnia. National Library of Medicine (US), National Center for Biotechnology Information, Bethesda
Ebert D, Lipsitch M, Mangin KL (2000) The effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites. Am Nat 156(5):459–477. https://doi.org/10.1086/303404
Fry WE, Goodwin SB (1997) Resurgence of the Irish potato famine fungus. Bioscience 47(6):363–371
Fuxa J, Richter A (1998) Repeated reversion of resistance to nucleopolyhedrovirus by Anticarsia gemmatalis. J Invertebr Pathol 71(2):159–164
Gandon S, Day T (2009) Evolutionary epidemiology and the dynamics of adaptation. Evolution: International Journal of Organic Evolution 63 (4):826–838
Hall SR, Sivars-Becker L, Becker C, Duffy MA, Tessier AJ, Caceres CE (2007) Eating yourself sick: transmission of disease as a function of foraging ecology. Ecol Lett 10(3):207–218. https://doi.org/10.1111/j.1461-0248.2006.01011.x
Hall SR, Becker CR, Duffy MA, Cáceres CE (2010) Variation in resource acquisition and use among host clones creates key epidemiological trade-offs. Am Nat 176(5):557–565. https://doi.org/10.1086/656523
Hall SR, Becker CR, Duffy MA, Caceres CE (2012) A power-efficiency trade-off in resource use alters epidemiological relationships. Ecology 93(3):645–656
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91(5):293–320
Jones CK (1995) Geometric singular perturbation theory. In: Johnson R (ed) Dynamical systems. Springer, Berlin, pp 44–118
Koskella B, Vergara D, Lively CM (2011) Experimental evolution of sexual host populations in response to sterilizing parasites. Evol Ecol Res 13(3):315–322
Kraaijeveld AR, Godfray HCJ (1997) Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389(6648):278–280
Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo L, Boch CA, Dobson AP, Dunham EJ, Fredensborg BL, Huspeni TC, Lorda J, Mababa L, Mancini FT, Mora AB, Pickering M, Talhouk NL, Torchin ME, Lafferty KD (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454(7203):515–518. https://doi.org/10.1038/nature06970
Milks ML, Myers JH, Leptich MK (2002) Costs and stability of cabbage looper resistance to a nucleopolyhedrovirus. Evol Ecol 16(4):369–385
Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E (2020) A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med 382(8):692–694
Penczykowski RM, Forde SE, Duffy MA (2011) Rapid evolution as a possible constraint on emerging infectious diseases. Freshw Biol 56(4):689–704
Philpott SM, Maldonado J, Vandermeer J, Perfecto I (2004) Taking trophic cascades up a level: behaviorally-modified effects of phorid flies on ants and ant prey in coffee agroecosystems. Oikos 105(1):141–147
Power AG, Mitchell CE (2004) Pathogen spillover in disease epidemics. Am Nat 164(S5):S79–S89
Rigby MC, Hechinger RF, Stevens L (2002) Why should parasite resistance be costly? Trends Parasitol 18(3):116–120
Roelke-Parker ME, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, O’Brien SJ, Pospischil A, Hofmann-Lehmann R, Lutz H (1996) A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379(6564):441
Strauss AT, Shocket MS, Civitello DJ, Hite JL, Penczykowski RM, Duffy MA, Caceres CE, Hall SR (2016) Habitat, predators, and hosts regulate disease in Daphnia through direct and indirect pathways. Ecol Monogr 86(4):393–411. https://doi.org/10.1002/ecm.1222
Strauss AT, Hite JL, Shocket MS, Cáceres CE, Duffy MA, Hall SR (2017) Rapid evolution rescues hosts from competition and disease but—despite a dilution effect—increases the density of infected hosts. Proc R Soc B 284(1868):20171970
Vale PF, Lafforgue G, Gatchitch F, Gardan R, Moineau S, Gandon S (2015) Costs of CRISPR-Cas-mediated resistance in Streptococcus thermophilus. Proc R Soc B Biol Sci 282(1812):20151270
Valtonen TM, Kleino A, Rämet M, Rantala MJ (2010) Starvation reveals maintenance cost of humoral immunity. Evol Biol 37(1):49–57
Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ (2010) Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA 107(21):9689–9694
Walsman JC, Strauss AT, Hall SR (2021) Parasite-driven cascades or hydra effects: susceptibility and foraging depression shape parasite–host–resource interactions. Funct Biol 36(5):1268–1278
Webster J, Woolhouse M (1999) Cost of resistance: relationship between reduced fertility and increased resistance in a snail–schistosome host–parasite system. Proc R Soc Lond B Biol Sci 266(1417):391–396
Zeller M, Koella JC (2017) The role of the environment in the evolution of tolerance and resistance to a pathogen. Am Nat 190(3):389–439
Acknowledgements
O. Schmidt assisted with the trait measurement assays. C. Lively, F. Bashey-Visser, and M. Wade provided valuable feedback on the manuscript.
Funding
This work was supported by NSF DEB 1353749 and 1655656 and NSF GRFP awards to J. Walsman and A. Strauss.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Walsman, J.C., Strauss, A.T., Hite, J.L. et al. A paradox of parasite resistance: disease-driven trophic cascades increase the cost of resistance, selecting for lower resistance with parasites than without them. Evol Ecol 37, 53–74 (2023). https://doi.org/10.1007/s10682-022-10203-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-022-10203-7