Abstract
We study a chemical reaction-diffusion model (the Brusselator) for pattern formation on developing plant tips. A family of spherical cap domains is used to represent tip flattening during development. Applied to conifer embryos, we model the chemical prepatterning underlying cotyledon (“seed leaf”) formation, and demonstrate the dependence of patterns on tip flatness, radius, and precursor concentrations. Parameters for the Brusselator in spherical cap domains can be chosen to give supercritical pitchfork bifurcations of patterned solutions of the nonlinear reaction-diffusion system that correspond to the cotyledon patterns that appear on the flattening tips of conifer embryos.







Similar content being viewed by others
References
Baker, R. E., & Maini, P. K. (2007). A mechanism for morphogen-controlled domain growth. J. Math. Biol., 54, 597–622.
Barrass, I., Crampin, E. G., & Maini, P. K. (2006). Mode transitions in a model reaction-diffusion system driven by domain growth and noise. Bull. Math. Biol., 68, 981–995.
Barreira, R., Elliott, C. M., & Madzvamuse, A. (2011). The surface finite element method for pattern formation on evolving biological surfaces. J. Math. Biol., 63, 1095–1119.
Bauer, H. F. (1986). Tables of the roots of the associated Legendre function with respect to degree. Math. Comput., 46, 601–602; S29–S41.
Bilsborough, G. D., Runions, A., Barkoulas, M., Jenkins, H. W., Hasson, A., Galinha, C., Laufs, P., Hay, A., Prusinkiewicz, P., & Tsiantis, M. (2011). Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. USA, 108, 3424–3429.
Carr, J. (1981). Applications of centre manifold theory. New York: Springer.
Cosgrove, D. J. (1996). Plant cell enlargement and the action of expansins. BioEssays, 18, 533–540.
Crampin, E. J., Hackborn, W. W., & Maini, P. K. (2002). Pattern formation in reaction-diffusion models with nonuniform domain growth. Bull. Math. Biol., 64, 747–769.
de Reuille, P. B., Bohn-Courseau, I., Ljung, K., Morin, H., Carraro, N., Godin, C., & Traas, J. (2006). Computer simulations reveal properties of the cell-cell signalling network at the shoot apex in Arabidopsis. Proc. Natl. Acad. Sci. USA, 103, 1627–1632.
Digiuni, S., Schellmann, S., Geier, F., Greese, B., Pesch, M., Wester, K., Dartan, B., Mach, V., Srinivas, B. P., Timmer, J., Fleck, C., & Hülskamp, M. (2008). A competitive complex formation mechanism underlies trichome patterning in Arabidopsis leaves. Mol. Syst. Biol., 4, 217.
Fleming, A. J., McQueen-Mason, S., Mandel, T., & Kuhlemeier, C. (1997). Induction of leaf primordia by the cell wall protein expansin. Science, 276, 1415–1418.
Fujita, H., Toyokura, K., Okada, K., & Kawaguchi, M. (2011). Reaction-diffusion mechanism in shoot apical meristem of plants. PLoS ONE, 6, e18243.
Garzón-Alvarado, D. A., Martinez, A. M. R., & Segrera, D. L. L. (2012). Appearance and formation of seed and pericarp may be explained by a reaction-diffusion mechanism? A mathematical modeling. Math. Comput. Model., 55, 853–860.
Gierer, A., & Meinhardt, H. (1972). A theory of biological pattern formation. Kybernetik, 12, 30–39.
Hakman, I., Hallberg, H., & Palovaara, J. (2009). The polar auxin transport inhibitor NPA impairs embryo morphology and increases the expression of an auxin efflux facilitator protein PIN during Picaea abies somatic embryo development. Tree Physiol., 29, 483–496.
Harrison, L. G. (2011). The shaping of life. Cambridge: Cambridge University Press.
Harrison, L. G., & Hillier, N. A. (1985). Quantitative control of Acetabularia morphogenesis by extracellular calcium: a test of kinetic theory. J. Theor. Biol., 114, 177–192.
Harrison, L. G., & Kolar, M. (1988). Coupling between reaction-diffusion prepattern and expressed morphogenesis, applied to desmids and dasyclads. J. Theor. Biol., 130, 493–515.
Harrison, L. G., & von Aderkas, P. (2004). Spatially quantitative control of the number of cotyledons in a clonal population of somatic embryos of hybrid larch Larix × leptoeuropaea. Ann. Bot., 93, 423–434.
Harrison, L. G., Snell, J., Verdi, R., Vogt, D. E., Zeiss, G. D., & Green, B. R. (1981). Hair morphogenesis in Acetabularia mediterranea: temperature-dependent spacing and models of morphogen waves. Protoplasma, 106, 211–221.
Harrison, L. G., Graham, K. T., & Lakowski, B. C. (1988). Calcium localization during Acetabularia whorl formation: evidence supporting a two-stage hierarchical mechanism. Development, 104, 255–262.
Harrison, L. G., Donaldson, G., Lau, W., Lee, M., Lin, B. P., Lohachitranont, S., Setyawati, I., & Yue, J. (1997). CaEGTA uncompetitively inhibits calcium activation of whorl morphogenesis in Acetabularia. Protoplasma, 196, 190–196.
Harrison, L. G., Wehner, S., & Holloway, D. M. (2001). Complex morphogenesis of surfaces: theory and experiment on coupling of reaction-diffusion to growth. Faraday Discuss., 120, 277–294.
Harrison, L. G., Adams, R. J., & Holloway, D. M. (2012). Dynamic regulation of growing domains for elongating and branching morphogenesis in plants. Biosystems, 109, 488–497.
Holloway, D. M. (2010). The role of chemical dynamics in plant morphogenesis. Biochem. Soc. Trans., 38, 645–650.
Holloway, D. M. (2012). The chemical kinetics of shape determination in plants. In V. Patel (Ed.), Chemical kinetics (pp. 203–226). Rijeka: InTech. Available at http://www.intechopen.com/articles/show/title/the-chemical-kinetics-of-shape-determination-in-plants.
Holloway, D. M., & Harrison, L. G. (1995). Order and localization in reaction-diffusion pattern. Physica A, 222, 210–233.
Holloway, D. M., & Harrison, L. G. (1999). Algal morphogenesis: modelling interspecific variation in Micrasterias with reaction-diffusion patterned catalysis of cell surface growth. Philos. Trans. R. Soc. Lond. B, 354, 417–433.
Holloway, D. M., & Harrison, L. G. (2008). Pattern selection in plants: coupling chemical dynamics to surface growth in three dimensions. Ann. Bot., 101, 361–374.
Iron, D., Ward, M. J., & Wei, J. C. (2001). The stability of spike solutions to the one-dimensional Gierer-Meinhardt model. Physica D, 150, 25–62.
Jönsson, H., Heisler, M. G., Reddy, G. V., Agrawal, V., Gor, V., Shapiro, B. E., Mjolsness, E., & Meyerowitz, E. M. (2005). Modeling the organization of the WUSCHEL expression domain in the shoot apical meristem. Bioinformatics, 21[Suppl.], i232–i240.
Jönsson, H., Heisler, M. G., Shapiro, B. E., Mjolsness, E., & Meyerowitz, E. M. (2006). An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. USA, 103, 1633–1638.
Lacalli, T. C. (1981). Dissipative structures and morphogenetic pattern in unicellular algae. Philos. Trans. R. Soc. Lond. B, 294, 547–588.
Madzvamuse, A., Gaffney, E. A., & Maini, P. K. (2010). Stability analysis of non-autonomous reaction-diffusion systems: the effects of growing domains. J. Math. Biol., 61, 113–164.
Meinhardt, H. (1982). Models of biological pattern formation. London: Academic Press. Available at http://www.eb.tuebingen.mpg.de/departments/former-departments/h-meinhardt/82-book/Bur82.htm.
Milani, P., Gholamirad, M., Traas, J., Arnéodo, A., Boudaoud, A., Argoul, F., & Hamant, O. (2011). In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. Plant J., 67, 1116–1123.
Miura, T., Shiota, K., Morriss-Kay, G., & Maini, P. K. (2006). Mixed-mode pattern in Doublefoot mutant mouse limb—Turing reaction-diffusion model on a growing domain during limb develpment. J. Theor. Biol., 240, 562–573.
Nagata, W., Harrison, L. G., & Wehner, S. (2003). Reaction-diffusion models of growing plant tips: bifurcations on hemispheres. Bull. Math. Biol., 65, 571–607.
Neville, A. A., Matthews, P. C., & Byrne, H. M. (2006). Interactions between pattern formation and domain growth. Bull. Math. Biol., 68, 1975–2003.
Palin, R., & Geitmann, A. (2012). The role of pectin in plant morphogenesis. Biosystems, 109, 397–402.
Palin, R. J. (2011). A comparison of cell wall properties of Arabidopsis thaliana. PhD thesis, University of Birmingham.
Peaucelle, A., Braybrook, S. A., Le Guillou, L., Bron, E., Kuhlemeier, C., & Höfte, H. (2011). Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol., 21, 1720–1726.
Plaza, R. G., Sánchez-Garduño, F., Padilla, P., Barrio, R. A., & Maini, P. K. (2004). The effect of growth and curvature on pattern formation. J. Dyn. Differ. Equ., 16, 1093–1121.
Prigogine, I., & Lefever, R. (1968). Symmetry-breaking instabilities in dissipative systems. II. J. Chem. Phys., 48, 1695–1700.
Routier-Kierzkowska, A.-L., Weber, A., Kochova, P., Felekis, D., Nelson, B. J., Kuhlemeier, C., & Smith, R. S. (2012). Cellular force microscopy for in vivo measurements of plant tissue mechanics. Plant Physiol., 158, 1514–1522.
Schnakenberg, J. (1979). Simple chemical reaction systems with limit cycle behaviour. J. Theor. Biol., 81, 389–400.
Smith, R. S., Guyomarch, S., Mandel, T., Reinhardt, D., Kuhlemeier, C., & Prusinkiewicz, P. (2006). A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. USA, 103, 1301–1306.
Turing, A. (1952). The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B, 237, 37–72.
van Mourik, S., Kaufmann, K., van Dijk, A. D. J., Angenent, G. C., Merks, R. M. H., & Molenaar, J. (2012). Simulation of organ patterning on the floral meristem using a polar auxin transport model. PLoS ONE, 7, e28762.
von Aderkas, P. (2002). In vitro phenotypic variation in larch cotyledon number. Int. J. Plant Sci., 163, 301–307.
Wolf, S., & Greiner, S. (2012). Growth control by cell wall pectins. Protoplasma, 249(Suppl. 2), S169–S175.
Acknowledgements
We thank NSERC (Canada), Isfahan University of Technology and British Columbia Institute of Technology for financial support, T.C. Lacalli for stimulating discussions, and P. von Aderkas for the images in Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Appendix: Reduction to the Bifurcation Equation
Appendix: Reduction to the Bifurcation Equation
In this Appendix, we outline the reduction of the Brusselator system (4)–(6) to the bifurcation equation (22),
and in particular the calculation of the cubic coefficient C. The calculation is standard, but it seems useful to give some details. For theoretical background, see, e.g., Carr (1981).
First, we choose parameter values so that all the eigenvalues σ of the linearized Brusselator (12)–(13) are real, and the largest eigenvalue σ max is 0 and corresponds to a normal mode with mode numbers m=m c and n=n c . For example, we could use the values (17)–(18), \(A=A^{\max}_{\sigma=0}=76.5466\), γ=γ c =0.5, R=R c =0.981310, then m c =5 and n c =1. We consider only vector functions U=(U(θ,ϕ,t),V(θ,ϕ,t)) that satisfy the homogeneous Dirichlet boundary conditions
Due to the symmetries (7), we may simplify our calculations by additionally restricting to functions of spherical polar coordinates (θ,ϕ) that are even in ϕ about ϕ=0. Then we can choose a real normal mode U (0) (the real part of a complex normal mode), and both the center eigenspace E c and the local center manifold \(W_{\mathrm{loc}}^{c}\) become one-dimensional, parameterized by the real variable x.
We solve the linear system
finding a specific normal mode
where
and
For any two vector functions U j =(U j (θ,ϕ,t),V j (θ,ϕ,t)), j=1, 2, we define their inner product as the integral
We solve the adjoint linear system
and find the adjoint normal mode
where
and
The normalization constant N (∗) for the adjoint normal mode is chosen so that
We can then define the projection P c of any vector function U onto the normal mode U (0) by
where
We can expand a solution U of (20) near the patternless solution U=0 in a Taylor series in the real variable x about x=0 as
where terms at second and higher orders in x are orthogonal to U (∗). Inserting this Taylor series expansion into the evolution equation (20), using the projection P c and collecting coefficients of powers of x, we find at second order in x that U (1) is the unique solution to the nonhomogeneous linear equation
At third order in x, we find that the cubic coefficient C in the bifurcation equation (22) is given by
Substituting the normal mode (23) into the right-hand side of the nonhomogeneous equation (24), we have
where
and then (24) can be solved for U (1) using infinite normal mode series expansions
In practice, if δ≠0, we approximate the solution U (1) using truncated, finite normal mode series expansions.
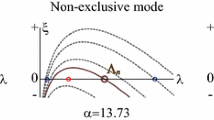
If we fix the parameters D X , D Y , a, b, B, and c, and then choose other parameters so that (16), (19), and \(A=A^{\max}_{\sigma=0}\) hold, then the coefficient δ in (26) can be expressed as a function of the remaining parameter d. It is easy to show in this case that if
for example, as in the parameter choices (17), then we have
and then using (26) the solution to (24) is
In this case, the formula (25) for the cubic coefficient C in the bifurcation equation simplifies to
and we have
Note that many expressions above such as u (0), v (0), N (0), k 5, M, u (∗), v (∗), and N (∗) depend on parameters, including m c and n c , but to simplify notation we have not explicitly shown the dependence. We used Maple to find numerical approximations of roots λ=λ m,n (γ), associated Legendre functions \(P^{m}_{\lambda}(\cos\theta)\), definite integrals, and (when δ≠0) coefficients for truncated, finite normal mode series expansions.
Rights and permissions
About this article
Cite this article
Nagata, W., Zangeneh, H.R.Z. & Holloway, D.M. Reaction-Diffusion Patterns in Plant Tip Morphogenesis: Bifurcations on Spherical Caps. Bull Math Biol 75, 2346–2371 (2013). https://doi.org/10.1007/s11538-013-9895-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-013-9895-5