Abstract
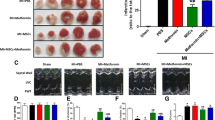
Type 1 diabetes mellitus (DM) is a strong risk factor for the development of diabetic cardiomyopathy (DCM) which is the leading cause of morbidity and mortality in the type 1 diabetic patients. Stem cells may act as a therapeutic agent for the repair of DCM. However, deteriorated functional abilities and survival of stem cells derived from type 1 diabetic subjects need to be overcome for obtaining potential outcome of the stem cell therapy. Diazoxide (DZ) a highly selective mitochondrial ATP-sensitive K+ channel opener has been previously shown to improve the ability of mesenchymal stem cells for the repair of heart failure. In the present study, we evaluated the effects of DZ preconditioning in improving the ability of streptozotocin-induced type 1 diabetes affected bone marrow-derived endothelial progenitor cells (DM-EPCs) for the repair of DCM in the type 1 diabetic rats. DM-EPCs were characterized by immunocytochemistry, flow cytometry, and reverse transcriptase PCR for endothelial cell-specific markers like vWF, VE cadherin, VEGFR2, PECAM, CD34, and eNOS. In vitro studies included preconditioning of DM-EPCs with 200 μM DZ for 30 min followed by exposure to either 200 μM H2O2 for 2 h (for oxidative stress induction) or 30 mM glucose media (for induction of hyperglycemic stress) for 48 h. Non-preconditioned EPCs with and without exposure to H2O2 and 30 mM high glucose served as controls. These cells were then evaluated for survival (by MTT and XTT cell viability assays), senescence, paracrine potential (by ELISA for VEGF), and alteration in gene expression [VEGF, stromal derived factor-1α (SDF-1α), HGF, bFGF, Bcl2, and Caspase-3]. DZ preconditioned DM-EPCs demonstrated significantly increased survival and VEGF release while reduced cell injury and senescence. Furthermore, DZ preconditioned DM-EPCs exhibited up-regulated expression of prosurvival genes (VEGF, SDF-1α, HGF, bFGF, and Bcl2) on exposure to H2O2, and VEGF and Bcl2 on exposure to hyperglycemia while down regulation of Caspase-3 gene. Eight weeks after type 1 diabetes induction, DZ preconditioned, and non-preconditioned DM-EPCs were transplanted into left ventricle of diabetic rats (at a dose of 2 × 106 DM-EPCs/70 μl serum free medium). After 4 weeks, DZ preconditioned DM-EPCs transplantation improved cardiac function as assessed by Millar’s apparatus. There was decrease in collagen content estimated by Masson’s trichrome and sirius red staining. Furthermore, reduced cell injury was observed as evidenced by decreased expression of Caspase-3 and increased expression of prosurvival genes Bcl2, VEGF, and bFGF by semi-quantitative real-time PCR. In conclusion, the present study demonstrated that DZ preconditioning enhanced EPCs survival under oxidative and hyperglycemic stress and their ability to treat DCM.







Similar content being viewed by others
References
Roglic G, Unwin N, Bennett PH et al (2005) The burden of mortality attributable to diabetes realistic estimates for the year 2000. Diabetes Care 28(9):2130–2135
Roger VL, Go AS, Lloyd-Jones DM et al (2012) Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125:e2–e220
Battiprolu PK, Gillette TG, Wang ZV et al (2010) Diabetic cardiomyopathy: mechanisms and therapeutic targets. Drug Discov Today Dis Mech 7:135–143
Boudina S, Abel E (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223
Federici M, Menghini R, Mauriello A et al (2002) Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106(4):466–472
He X, Kan H, Cai L et al (2009) Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol 46:47–58
Giugliano D, Ceriello A, Paolisso G (1995) Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism 44:363–368
Meigs JB, Larson MG, Fox CS et al (2007) Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diabetes Care 30:2529–2535
Cai L, Kang YJ (2001) Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol 1:181–193
Devereux RB, Roman MJ, Paranicas M et al (2000) Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 101:2271–2276
Hohenstein B, Kuo MC, Addabbo F et al (2013) Enhanced progenitor cell recruitment and endothelial repair after selective endothelial injury of the mouse kidney. Am J Physiol Ren Physiol 298:1504–1514
Allegra A, Coppolino G, Bolignano D et al (2009) Endothelial progenitor cells: pathogenetic role and therapeutic perspectives. J Nephrol 22:463–475
Sen S, McDonald SP, Coates PT et al (2011) Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (Lond) 120:263–283
Alev C, Li M, Asahara T (2011) Endothelial progenitor cells: a novel tool for the therapy of ischemic diseases. Antioxid Redox Signal 15:949–965
Grisar JC, Haddad F, Gomari FA et al (2011) Endothelial progenitor cells in cardiovascular disease and chronic inflammation: from biomarker to therapeutic agent. Biomark Med 5:731–744
Kawamoto A, Tkebuchava T, Yamaguchi J et al (2003) Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107:461–468
Werner N, Junk S, Laufs U et al (2003) Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 93:17–24
Ohta T, Kikuta K, Imamura H et al (2006) Administration of ex vivo-expanded bone marrow-derived endothelial progenitor cells attenuates focal cerebral ischemia-reperfusion injury in rats. Neurosurgery 59:679–686
Grapensparr L, Olerud J, Vasylovska S et al (2011) The therapeutic role of endothelial progenitor cells in Type 1 diabetes mellitus. Regen Med 6:599–605
Georgescu A (2011) Vascular dysfunction in diabetes: the endothelial progenitor cells as new therapeutic strategy. World J Diabetes 2:92–97
Kim KA, Shin YJ, Kim JH et al (2012) Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch Pharmacal Res 35:223–234
Qu Z, Balkir L, Van Deutekom JC et al (1998) Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol 142:1257–1267
Niagara MI, Haider H, Jiang S et al (2007) Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res 100:545–555
Rajapakse N, Kis B, Horiguchi T et al (2003) Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. J Neurosci Res 73:206–214
Loomans CJ, de Koning EJ, Staal FJ et al (2004) Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53(1):195–199
Khan M, Akhtar S, Mohsin S et al (2011) Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev 20:67–75
Biase FH, Franco MM, Goulart LR et al (2002) Protocol for extraction of genomic DNA from swine solid tissues. Genet Mol Biol 25(3):313–315
Cheng Y, Guo S, Liu G et al (2012) Transplantation of bone marrow-derived endothelial progenitor cells attenuates myocardial interstitial fibrosis and cardiac dysfunction in streptozotocin-induced diabetic rats. Int J Mol Med 30:870–876
Jujo K, Li M, Losordo DW (2008) Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol 45(4):530–544
Yoon YS, Uchida S, Masuo O et al (2005) Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 111(16):2073–2085
Jesmin S, Sakuma I, Hattori Y et al (2003) Role of angiotensin II in altered expression of molecules responsible for coronary matrix remodeling in insulin-resistant diabetic rats. Arterioscler Thromb Vasc Biol 23:2021–2026
Haidara MA, Yassin HZ, Rateb M et al (2006) Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmacol 4:215–227
Pfister O, Lorenz V, Oikonomopoulos A et al (2013) Flt3 activation improves post-myocardial infarction remodeling involving a cytoprotective effect on cardiomyocytes. J Am Coll Cardiol 63(10):1011–1019
Lee D, Bae S, Ke Q et al (2013) Hydrogen peroxide-responsive copolyoxalate nanoparticles for detection and therapy of ischemia-reperfusion injury. J Control Release 172(3):1102–1110
Dhanasekaran M, Indumathi S, Rajkumar JS et al (2013) Effect of high glucose on extensive culturing of mesenchymal stem cells derived from subcutaneous fat, omentum fat and bone marrow. Cell Biochem Funct 31:20–29
Westermann D, Van Linthout S, Dhayat S et al (2007) Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes 56:1834–1841
Afzal MR, Haider HKh, Idris NM et al (2010) Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid Redox Signal 12(6):693–702
Cui X, Wang H, Guo H et al (2010) Transplantation of mesenchymal stem cells preconditioned with diazoxide, a mitochondrial ATP-sensitive potassium channel opener, promotes repair of myocardial infarction in rats. Tohoku J Exp Med 220(2):139–147
Spyridopoulos I, Brogi E, Kearney M et al (1997) Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-alpha: balance between growth and death signals. J Mol Cell Cardiol 29:1321–1330
Dimmeler S, Zeiher AM (2000) Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res 87:434–439
Urbich C, Aicher A, Heeschen C et al (2005) Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 39:733–742
Nayak G, Cooper GM (2012) p53 is a major component of the transcriptional and apoptotic program regulated by PI 3-kinase/Akt/GSK3 signaling. Cell Death Dis 3:e400
Shao SX, Zhang L, Chen HX et al (2012) Diazoxide pretreatment enhances L6 skeletal myoblast survival and inhibits apoptosis induced by hydrogen peroxide. Anat Rec (Hoboken) 295(4):632–640
Huang Q, Bu S, Yu Y et al (2007) Diazoxide prevents diabetes through inhibiting pancreatic beta-cells from apoptosis via Bcl-2/Bax rate and p38-beta mitogen-activated protein kinase. Endocrinology 148(1):81–91
Haider KH, Idris NM, Kim HW et al (2010) MicroRNA-21 is a key determinant in IL-11/Stat3 anti-apoptotic signalling pathway in preconditioning of skeletal myoblasts. Cardiovasc Res 88(1):168–178
Nilakantan V, Liang H, Mortensen J et al (2010) Variable effects of the mitoKATP channel modulators diazoxide and 5-HD in ATP-depleted renal epithelial cells. Mol Cell Biochem 335:211–222
Zhang H, Zhao D, Wang Z et al (2010) Diazoxide preconditioning alleviates caspase-dependent and caspase-independent apoptosis induced by anoxia-reoxygenation of PC12 cells. J Biochem 148(4):413–421
Takashi E, Wang Y, Ashraf M (1999) Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res 85:1146–1153
Wang Y, Takashi E, Xu M et al (2001) Downregulation of protein kinase C inhibits activation of mitochondrial K(ATP) channels by diazoxide. Circulation 104:85–90
Kudo M, Wang Y, Xu M et al (2002) Adenosine A(1) receptor mediates late preconditioning via activation of PKC-delta signaling pathway. Am J Physiol Heart Circ Physiol 283:H296–H301
Dzeja PP, Bast P, Ozcan C et al (2003) Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol 284:H1048–H1056
Virgili N, Mancera P, Wappenhans B (2013) K(ATP) channel opener diazoxide prevents neurodegeneration: a new mechanism of action via antioxidative pathway activation. PLoS One 8(9):e75189
Hole LD, Larsen TH, Fossan KO et al (2014) Diazoxide protects against doxorubicin-induced cardiotoxicity in the rat. BMC Pharmacol Toxicol 15:28
Hadi N, Yousif NG, Al-amran FG et al (2012) Vitamin E and telmisartan attenuates doxorubicin induced cardiac injury in rat through down regulation of inflammatory response. BMC Cardiovasc Disord 12:63
Rungwerth K, Schindler U, Gerl M et al (2004) Inhibition of Na+–H+ exchange by cariporide reduces inflammation and heart failure in rabbits with myocardial infarction. Br J Pharmacol 142:1147–1154
Huynh K, McMullen JR, Julius TL et al (2010) Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes 59:1512–1520
Kajstura J, Fiordaliso F, Andreoli AM et al (2001) IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 50:1414–1424
Khan M, Ali F, Mohsin S et al (2013) Preconditioning diabetic mesenchymal stem cells with myogenic medium increases their ability to repair diabetic heart. Stem Cell Res Ther 4:58
Huang ZG, Jin Q, Fan M et al (2013) Myocardial remodeling in diabetic cardiomyopathy associated with cardiac mast cell activation. PLoS One 8(3):e60827
Chen J, Cha-Molstad H, Szabo A et al (2009) Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab 296:e1133–e1139
Li JH, Zhang N, Wang JA (2011) Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J Endocrinol Investig 31:103–110
Acknowledgments
This work was supported, in part, by a Grant from the Higher Education Commission, Islamabad, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests exist.
Rights and permissions
About this article
Cite this article
Ali, M., Mehmood, A., Anjum, M.S. et al. Diazoxide preconditioning of endothelial progenitor cells from streptozotocin-induced type 1 diabetic rats improves their ability to repair diabetic cardiomyopathy. Mol Cell Biochem 410, 267–279 (2015). https://doi.org/10.1007/s11010-015-2560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2560-6