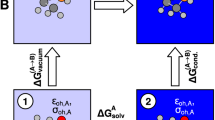
Abstract
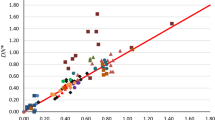
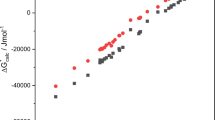
The chemical hardness of a solvent can play a decisive role in solubility and reactivity in solution. Several empirical scales quantifying solvent softness have been proposed. We explore whether computed properties of solvent molecules can reproduce these experimental scales. Our “orbital overlap distance” quantifying the size of orbitals at a molecule’s surface effectively reproduces the Marcus μ-scale of solvent softness. The orbital overlap distance predicts that the surface of chemically hard solvent molecules is dominated by compact orbitals possessing a small orbital overlap distance. In contrast, the surface of chemically soft solvent molecules has a larger contribution from diffuse orbitals and a larger orbital overlap distance. Other conceptual density functional theory descriptors, including the global hardness and electronegativity, can also reproduce the Marcus scale. We further introduce a “solvent versatility” RMSD Dsurf scale quantifying variations in the surface orbital overlap distance. “Good” solvents such as DMSO, which combine chemically “hard” and “soft” sites within a single molecule, possess a large RMSD Dsurf. We conclude by applying this approach to predict the Marcus μ-parameters for widely-used ionic liquids and ionic liquid–cosolvent systems.





Similar content being viewed by others
References
Lewis, G.N.: Valence and the Structure of Atoms and Molecules. Dover Publications, New York (1923)
Lewis, G.N.: Acids and bases. J. Franklin Inst. 226(3), 293–313 (1938). https://doi.org/10.1016/S0016-0032(38)91691-6
Chen, T., Hefter, G., Marcus, Y.: Relationships among solvent softness scales. J. Solution Chem. 29(3), 201–216 (2000). https://doi.org/10.1023/a:1005114615767
Pearson, R.G.: Hard and soft acids and bases. J. Am. Chem. Soc. 85(22), 3533–3539 (1963). https://doi.org/10.1021/ja00905a001
Pearson, R.G.: Acids and bases. Science 151(3707), 172–177 (1966). https://doi.org/10.1126/science.151.3707.172
Pearson, R.G.: Hard and soft acids and bases—the evolution of a chemical concept. Coord. Chem. Rev. 100, 403–425 (1990). https://doi.org/10.1016/0010-8545(90)85016-L
Reichardt, C., Welton, T.: Solvents and Solvent Effects in Organic Chemistry. Wiley, Hoboken (2011)
Bogachev, N.A., Gorbunov, A.O., Tikhomirova, A.A., Pushikhina, O.S., Skripkin, M.Y., Nikolskii, A.B.: Solubility of d-elements salts in organic and aqueous-organic solvents: I. Copper, cobalt, and cadmium sulfates. Russ. J. Gen. Chem. 85(11), 2509–2512 (2015). https://doi.org/10.1134/s107036321511002x
Gorbunov, A.O., Tsyrulnikov, N.A., Tikhomirova, A.A., Bogachev, N.A., Skripkin, M.Y., Nikolskii, A.B., Pestova, O.N.: Solubility of d-element salts in organic and aqueous–organic solvents: II. Effect of halocomplex formation on solubility of cobalt bromide and chloride and nickel chloride. Russ. J. Gen. Chem. 86(4), 771–777 (2016). https://doi.org/10.1134/s1070363216040022
Payehghadr, M., Hashemi, S.E.: Solvent effect on complexation reactions. J. Incl. Phenom. Macrocycl. Chem. 89(3), 253–271 (2017). https://doi.org/10.1007/s10847-017-0759-8
Claisen, L.: Über C-alkylierung (Kernalkylierung) von Phenolen. Angew. Chem. 36(65), 478–479 (1923). https://doi.org/10.1002/ange.19230366502
Kornblum, N., Berrigan, P.J., le Noble, W.J.: Chemical effects arising from selective solvation: selective solvation as a factor in the alkylation of ambident anions. J. Am. Chem. Soc. 82(5), 1257–1258 (1960). https://doi.org/10.1021/ja01490a063
Kornblum, N., Berrigan, P.J., Le Noble, W.J.: Solvation as a factor in the alkylation of ambident anions: the importance of the hydrogen bonding capacity of the solvent. J. Am. Chem. Soc. 85(8), 1141–1147 (1963). https://doi.org/10.1021/ja00891a024
Hughes, E.D., Ingold, C.K.: Mechanism of substitution at a saturated carbon atom. Part IV. A discussion of constitutional and solvent effects on the mechanism, kinetics, velocity, and orientation of substitution. J. Chem. Soc. (1935). https://doi.org/10.1039/JR9350000244
Hughes, E.D.: Mechanism and kinetics of substitution at a saturated carbon atom. Trans. Faraday Soc. 37, 603–631 (1941). https://doi.org/10.1039/TF9413700603
Cooper, K.A., Dhar, M.L., Hughes, E.D., Ingold, C.K., MacNulty, B.J., Woolf, L.I.: Mechanism of elimination reactions. Part VII. Solvent effects on rates and product-proportions in uni- and bi-molecular substitution and elimination reactions of alkyl halides and sulphonium salts in hydroxylic solvents. J. Chem. Soc. (1948). https://doi.org/10.1039/JR9480002043
Greenwald, R., Chaykovsky, M., Corey, E.J.: The Wittig reaction using methylsulfinyl carbanion-dimethyl sulfoxide 1. J. Org. Chem. 28(4), 1128–1129 (1963). https://doi.org/10.1021/jo01039a502
Laoire, C.O., Mukerjee, S., Abraham, K.M., Plichta, E.J., Hendrickson, M.A.: Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium−air battery. J. Phys. Chem. C 114(19), 9178–9186 (2010). https://doi.org/10.1021/jp102019y
Marcus, Y.: The use of chemical probes for the characterization of solvent mixtures. Part 2. Aqueous mixtures. J. Chem. Soc. Perkin Trans. 2(8), 1751–1758 (1994). https://doi.org/10.1039/P29940001751
Allen, C.J., Hwang, J., Kautz, R., Mukerjee, S., Plichta, E.J., Hendrickson, M.A., Abraham, K.M.: Oxygen reduction reactions in ionic liquids and the formulation of a general ORR mechanism for Li–air batteries. J. Phys. Chem. C 116(39), 20755–20764 (2012). https://doi.org/10.1021/jp306718v
Peppel, T., Köckerling, M., Geppert-Rybczyńska, M., Ralys, R.V., Lehmann, J.K., Verevkin, S.P., Heintz, A.: Low-viscosity paramagnetic ionic liquids with doubly charged [Co(NCS)4]2− Ions. Angew. Chem. Int. Ed. 49(39), 7116–7119 (2010). https://doi.org/10.1002/anie.201000709
Tsurumaki, A., Trequattrini, F., Palumbo, O., Panero, S., Paolone, A., Navarra, M.A.: The effect of ether-functionalisation in ionic liquids analysed by DFT calculation, infrared spectra, and Kamlet-Taft parameters. PCCP 20(12), 7989–7997 (2018). https://doi.org/10.1039/C7CP08134K
Tsurumaki, A., Kagimoto, J., Ohno, H.: Properties of polymer electrolytes composed of poly(ethylene oxide) and ionic liquids according to hard and soft acids and bases theory. Polym. Adv. Technol. 22(8), 1223–1228 (2011). https://doi.org/10.1002/pat.1931
Marcus, Y.: Linear solvation energy relationships: a scale describing the “softness” of solvents. J. Phys. Chem. 91(16), 4422–4428 (1987). https://doi.org/10.1021/j100300a044
Persson, I., Sandström, M., Goggin, P.L.: On the coordinating properties of some solvents. A vibrational spectroscopic study of mercury(II) halides and antimony(V) chloride in solution; new concepts for Lewis basicity scales of solvents. Inorg. Chim. Acta 129(2), 183–197 (1987). https://doi.org/10.1016/S0020-1693(00)86662-1
Laurence, C., Queignec-Cabanetos, M., Dziembowska, T., Queignec, R., Wojtkowiak, B.: 1-Iodoacetylenes 1 Spectroscopic evidence of their complexes with Lewis bases. A spectroscopic scale of soft basicity. J. Am. Chem. Soc. 103(10), 2567–2573 (1981). https://doi.org/10.1021/ja00400a014
Gritzner, G.: Solvent effects on half-wave potentials. J. Phys. Chem. 90(21), 5478–5485 (1986). https://doi.org/10.1021/j100412a116
Gutmann, V., Wychera, E.: Coordination reactions in non aqueous solutions: the role of the donor strength. Inorg. Nucl. Chem. Lett. 2(9), 257–260 (1966). https://doi.org/10.1016/0020-1650(66)80056-9
Maria, P.C., Gal, J.F.: A Lewis basicity scale for nonprotogenic solvents: enthalpies of complex formation with boron trifluoride in dichloromethane. J. Phys. Chem. 89(7), 1296–1304 (1985). https://doi.org/10.1021/j100253a048
Oshima, T., Arikata, S., Nagai, T.: Solvent effects in the reaction of diazodiphenylmethane with tetracycanoethylene: a new empirical parameter of solvent basicity. J. Chem. Res. 2, 204–205 (1981)
Dong, D.C., Winnik, M.A.: The Py scale of solvent polarities. Solvent effects on the vibronic fine structure of pyrene fluorescence and empirical correlations with Et and Y values. Photochem. Photobiol. 35(1), 17–21 (1982). https://doi.org/10.1111/j.1751-1097.1982.tb03805.x
Katritzky, A.R., Tamm, T., Wang, Y., Sild, S., Karelson, M.: QSPR treatment of solvent scales. J. Chem. Inf. Comput. Sci. 39(4), 684–691 (1999). https://doi.org/10.1021/ci980225h
Gutmann, V.: Empirical parameters for donor and acceptor properties of solvents. Electrochim. Acta 21(9), 661–670 (1976). https://doi.org/10.1016/0013-4686(76)85034-7
Persson, I.: Solvation and complex formation in strongly solvating solvents. Pure Appl. Chem. 58(8), 1153–1161 (1986)
Sandström, M., Persson, I., Persson, P.: A study of solvent electron-pair donor ability and Lewis basicity scales. Acta Chem. Scand 44(7), 653–675 (1990). https://doi.org/10.3891/acta.chem.scand.44-0653
Geerlings, P., De Proft, F., Langenaeker, W.: Conceptual density functional theory. Chem. Rev. 103(5), 1793–1874 (2003). https://doi.org/10.1021/cr990029p
Parr, R.G., Pearson, R.G.: Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105(26), 7512–7516 (1983). https://doi.org/10.1021/ja00364a005
Ayers, P.W.: An elementary derivation of the hard/soft-acid/base principle. J. Chem. Phys. 122(14), 141102 (2005). https://doi.org/10.1063/1.1897374
Yang, W., Parr, R.G.: Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc. Natl. Acad. Sci. U.S.A. 82(20), 6723–6726 (1985). https://doi.org/10.1073/pnas.82.20.6723
Jayakumar, N., Kolandaivel, P.: Studies of isomer stability using the maximum hardness principle (MHP). Int. J. Quantum Chem. 76(5), 648–655 (2000). https://doi.org/10.1002/(SICI)1097-461X(2000)76:5%3c648:AID-QUA7%3e3.0.CO;2-Y
Padmanabhan, J., Parthasarathi, R., Subramanian, V., Chattaraj, P.K.: Molecular structure, reactivity, and toxicity of the complete series of chlorinated benzenes. J. Phys. Chem. A 109(48), 11043–11049 (2005). https://doi.org/10.1021/jp0538621
Senthilkumar, K., Kolandaivel, P.: Hartree–Fock and density functional theory studies on ionization and fragmentation of halomethane molecules by positron impact. Mol. Phys. 100(24), 3817–3822 (2002). https://doi.org/10.1080/00268970210161939
Shankar, R., Senthilkumar, K., Kolandaivel, P.: Calculation of ionization potential and chemical hardness: a comparative study of different methods. Int. J. Quantum Chem. 109(4), 764–771 (2009). https://doi.org/10.1002/qua.21883
Geerlings, P., De Proft, F.: HSAB principle: applications of its global and local forms in organic chemistry. Int. J. Quantum Chem. 80(2), 227–235 (2000). https://doi.org/10.1002/1097-461X(2000)80:2%3c227:AID-QUA17%3e3.0.CO;2-N
Langenaeker, W., de Proft, F., Geerlings, P.: Development of local hardness-related reactivity indices: their application in a study of the SE at monosubstituted benzenes within the HSAB context. J. Phys. Chem. 99(17), 6424–6431 (1995). https://doi.org/10.1021/j100017a022
Mendez, F., Garcia-Garibay, M.A.: A Hard−soft acid−base and DFT analysis of singlet−triplet gaps and the addition of singlet carbenes to alkenes. J. Org. Chem. 64(19), 7061–7066 (1999). https://doi.org/10.1021/jo990584r
Chattaraj, P.K., Fuentealba, P., Gómez, B., Contreras, R.: Woodward−Hoffmann rule in the light of the principles of maximum hardness and minimum polarizability: DFT and ab initio SCF studies. J. Am. Chem. Soc. 122(2), 348–351 (2000). https://doi.org/10.1021/ja992337a
Balawender, R., Komorowski, L., De Proft, F., Geerlings, P.: Derivatives of molecular valence as a measure of aromaticity. J. Phys. Chem. A 102(48), 9912–9917 (1998). https://doi.org/10.1021/jp982447o
Sarmah, P., Deka, R.C.: Solvent effect on the reactivity of CIS-platinum(II) complexes: a density functional approach. Int. J. Quantum Chem. 108(8), 1400–1409 (2008). https://doi.org/10.1002/qua.21635
Panina, N.S., Calligaris, M.: Density functional study of linkage isomerism in dimethyl sulfoxide Ru(III) and Rh(III) complexes. Inorg. Chim. Acta 334, 165–171 (2002). https://doi.org/10.1016/S0020-1693(02)00752-1
Bania, K.K., Deka, R.C.: Influence of Zeolite framework on the structure, properties, and reactivity of cobalt phenanthroline complex: a combined experimental and computational study. J. Phys. Chem. C 115(19), 9601–9607 (2011). https://doi.org/10.1021/jp2003672
Islam, N., Kaya, S.: Conceptual Density Functional Theory and Its Application in the Chemical Domain. Apple Academic Press, New York (2018)
Frau, J., Hernández-Haro, N., Glossman-Mitnik, D.: Computational prediction of the pKas of small peptides through conceptual DFT descriptors. Chem. Phys. Lett. 671, 138–141 (2017). https://doi.org/10.1016/j.cplett.2017.01.038
Putz, M.V., Mingos, D.M.P.: Applications of Density Functional Theory to Chemical Reactivity. Springer, Berlin (2013)
Pearson, R.G.: Chemical Hardness: Applications from Molecules to Solids. Wiley, Hoboken (1998)
Bader, R.F.W., Carroll, M.T., Cheeseman, J.R., Chang, C.: Properties of atoms in molecules: atomic volumes. J. Am. Chem. Soc. 109(26), 7968–7979 (1987). https://doi.org/10.1021/ja00260a006
Politzer, P., Murray, J.S.: σ-holes and π-holes: Similarities and differences. J. Comput. Chem. 39(9), 464–471 (2018). https://doi.org/10.1002/jcc.24891
Kolář, M.H., Hobza, P.: Computer modeling of halogen bonds and other σ-hole interactions. Chem. Rev. 116(9), 5155–5187 (2016). https://doi.org/10.1021/acs.chemrev.5b00560
Mehmood, A., Janesko, B.G.: An orbital-overlap complement to atomic partial charge. Angew. Chem. Int. Ed. 56(24), 6878–6881 (2017). https://doi.org/10.1002/anie.201702715
Janesko, B.G., Wiberg, K.B., Scalmani, G., Frisch, M.J.: Electron delocalization range in atoms and on molecular surfaces. J. Chem. Theory Comput. 12(7), 3185–3194 (2016). https://doi.org/10.1021/acs.jctc.6b00343
Gritzner, G., Auinger, M.: Statistical analysis of Gibbs energies of transfer of cations and soft solvent parameters. Acta Chim. Slov. 56(1), 86–94 (2009)
Armand, M., Endres, F., MacFarlane, D.R., Ohno, H., Scrosati, B.: Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 8, 621 (2009). https://doi.org/10.1038/nmat2448
Wilkes, J.S.: A short history of ionic liquids—from molten salts to neoteric solvents. Green Chem. 4(2), 73–80 (2002). https://doi.org/10.1039/B110838G
Van Aken, K.L., Beidaghi, M., Gogotsi, Y.: Formulation of ionic-liquid electrolyte to expand the voltage window of supercapacitors. Angew. Chem. Int. Ed. 54(16), 4806–4809 (2015). https://doi.org/10.1002/anie.201412257
Hayes, R., Warr, G.G., Atkin, R.: Structure and nanostructure in ionic liquids. Chem. Rev. 115(13), 6357–6426 (2015). https://doi.org/10.1021/cr500411q
MacFarlane, D.R., Tachikawa, N., Forsyth, M., Pringle, J.M., Howlett, P.C., Elliott, G.D., Davis, J.H., Watanabe, M., Simon, P., Angell, C.A.: Energy applications of ionic liquids. Energy Environ. Sci. 7(1), 232–250 (2014). https://doi.org/10.1039/C3EE42099J
Zhao, Y., Wang, J., Wang, H., Li, Z., Liu, X., Zhang, S.: Is there any preferential interaction of ions of ionic liquids with DMSO and H2O? A comparative study from MD simulation. J. Phys. Chem. B 119(22), 6686–6695 (2015). https://doi.org/10.1021/acs.jpcb.5b01925
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M.J., Heyd, J., Brothers, E.N., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A.P., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, N.J., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09. Gaussian Inc, Wallingford, CT (2009)
Becke, A.D.: A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 98(2), 1372–1377 (1993). https://doi.org/10.1063/1.464304
Lee, C., Yang, W., Parr, R.G.: Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37(2), 785–789 (1988). https://doi.org/10.1103/PhysRevB.37.785
Francl, M.M., Pietro, W.J., Hehre, W.J., Binkley, J.S., Gordon, M.S., DeFrees, D.J., Pople, J.A.: Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 77(7), 3654–3665 (1982). https://doi.org/10.1063/1.444267
Hariharan, P.C., Pople, J.A.: Accuracy of AH AnD equilibrium geometries by single determinant molecular orbital theory. Mol. Phys. 27(1), 209–214 (1974). https://doi.org/10.1080/00268977400100171
Hariharan, P.C., Pople, J.A.: The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28(3), 213–222 (1973). https://doi.org/10.1007/bf00533485
Hehre, W.J., Ditchfield, R., Pople, J.A.: Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56(5), 2257–2261 (1972). https://doi.org/10.1063/1.1677527
Ditchfield, R., Hehre, W.J., Pople, J.A.: Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54(2), 724–728 (1971). https://doi.org/10.1063/1.1674902
Lu, T., Chen, F.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33(5), 580–592 (2012). https://doi.org/10.1002/jcc.22885
Lu, T., Chen, F.: Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. Model. 38, 314–323 (2012). https://doi.org/10.1016/j.jmgm.2012.07.004
Dogonadze, R.R., Kalman, E., Kornyshev, A.A.: The Chemical Physics of Solvation: Theory of Solvation. Elsevier, Amsterdam (1985)
Fujii, K., Fujimori, T., Takamuku, T., Kanzaki, R., Umebayashi, Y., Ishiguro, S.-I.: Conformational equilibrium of bis(trifluoromethanesulfonyl) imide anion of a room-temperature ionic liquid: Raman spectroscopic study and DFT calculations. J. Phys. Chem. B 110(16), 8179–8183 (2006). https://doi.org/10.1021/jp0612477
Umebayashi, Y., Fujimori, T., Sukizaki, T., Asada, M., Fujii, K., Kanzaki, R., Ishiguro, S.-I.: Evidence of conformational equilibrium of 1-ethyl-3-methylimidazolium in its ionic liquid salts: Raman spectroscopic study and quantum chemical calculations. J. Phys. Chem. A 109(40), 8976–8982 (2005). https://doi.org/10.1021/jp053476j
Singh, D.K., Cha, S., Nam, D., Cheong, H., Joo, S.-W., Kim, D.: Raman spectroscopic study on alkyl chain conformation in 1-butyl-3-methylimidazolium ionic liquids and their aqueous mixtures. ChemPhysChem 17(19), 3040–3046 (2016). https://doi.org/10.1002/cphc.201600485
Xu, A., Zhang, Y., Zhao, Y., Wang, J.: Cellulose dissolution at ambient temperature: role of preferential solvation of cations of ionic liquids by a cosolvent. Carbohydr. Polym. 92(1), 540–544 (2013). https://doi.org/10.1016/j.carbpol.2012.09.028
Zhao, Y., Liu, X., Wang, J., Zhang, S.: Insight into the cosolvent effect of cellulose dissolution in imidazolium-based ionic liquid systems. J. Phys. Chem. B 117(30), 9042–9049 (2013). https://doi.org/10.1021/jp4038039
Gupta, K.M., Jiang, J.: Cellulose dissolution and regeneration in ionic liquids: a computational perspective. Chem. Eng. Sci. 121, 180–189 (2015). https://doi.org/10.1016/j.ces.2014.07.025
Acknowledgements
This work was supported by the National Science Foundation (DMR-1505343).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10953_2020_973_MOESM1_ESM.docx
Supplementary file1 (DOCX 1036 kb) The following quantities are reported in the supplementary material. HOMO–LUMO surface plots of selective solvents, correlation between μ and 1/Gap, mean Dsurf and 1/Gap and μ and 1/Gap. Experimental μ values of solvent and their calculated values of mean Dsurf and RMSD Dsurf, with and without neutral regions. Calculated values of 1/Gap and 1/(I – A) for all solvents. Calculated values for ionic liquids. Experimental values of Ds and DN used in this study. Basis set dependence of Dsurf and RMSD Dsurf.
Rights and permissions
About this article
Cite this article
Mehmood, A., Janesko, B.G. Extending the Marcus μ-Scale of Solvent Softness Using Conceptual Density Functional Theory and the Orbital Overlap Distance: Method and Application to Ionic Liquids. J Solution Chem 49, 614–628 (2020). https://doi.org/10.1007/s10953-020-00973-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-00973-5