Abstract
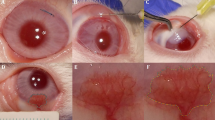
To evaluate the effects of various doses of subconjunctival bevacizumab injections in the treatment of patients with corneal neovascularization. During the 6-month-follow-up, no significant ocular or systemic adverse events were observed related to the subconjunctival bevacizumab injection. In Group 1, the total area of corneal neovascularization before injection was 14.8 ± 3.2 % of the corneal surface and 10.2 ± 2.8 % 6 months after injection (p < 0.01). The mean decrease in Group 1 was 32.0 ± 3.0 %. In Group 2, the total area of corneal neovascularization before and 6 months after the injection was 14.2 ± 2.5 and 9.8 ± 2.3 %, respectively (p < 0.01). The mean decrease in Group 2 was 31.0 ± 2.3 %. The difference between the two groups was not statistically significant (p > 0.05). Twenty-four eyes of 24 patients with corneal neovascularization who were treated with a subconjunctival injection of bevacizumab were included in this retrospective study. Fourteen eyes were treated with 2.5 mg/0.1 ml (Group 1), and 10 eyes were treated with 5.0 mg/0.2 ml (Group 2) of subconjunctival bevacizumab. Digital photographs of the cornea were used to determine the area of corneal neovascularization before injection and at 1 month, 3 months, and 6 months after treatment. Subconjunctival injection of bevacizumab is well tolerated and associated with a partial regression of corneal neovascularization. The efficacy of this treatment is not correlated to the injection dose.




Similar content being viewed by others
References
Epstein RJ, Stulting RD, Hendricks RL, Harris DM (1987) Corneal neovascularization. Pathogenesis and inhibition. Cornea 64:250–257
Chang JH, Gabison EE, Kato T, Azar DT (2001) Corneal neovascularization. Curr Opin Ophthalmol 12:242–249
Kabbnivar FI, Meropol NJ, Lieberman G, Griffing S, Bergsland E (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60–65
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Rosenfeld PJ, Schwartz SD, Blumenkranz MS, Miller JW, Haller JA, Reimann JD, Greene WL, Shams N (2005) Maximum tolerated dose of a humanized anti-vaskular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 112:1048–1053
Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS (2005) Systemic bevacizumab (Avastin) therapy for neovascular age related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 112:1035–1047
Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ (2006) Intravitreal bevacizumab (Avastin) for neovascular age related macular degeneration. Ophthalmology 113:363–372
Nguyen QD, Shah S, Tatlipinar S, Do DV, Anden EV, Campochiaro PA (2005) Bevacizumab suppresses choroidal neovascularization caused by pathological myopia. Br J Ophthalmol 89:1368–1370
Manzano RP, Peyman GA, Khan P, Carvounis PE, Kivilcim M, Reen M, Lake JC, Chevez-Barrios P (2007) Inhibition of experimental corneal neovascularısatıon by bevacizumab (Avastin). Br J Ophthalmol 91:804–807
Kim TI, Kim SW, Kim S, Kim T, Kim EK (2008) Inhibition of experimental corneal neovascularization by using subconjunctival injection of bevacizumab (Avastin). Cornea 27:349–352
Hashemian MN, Zare MA, Rahimi F, Mohammadpour M (2011) Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea 30:215–218
Vassileva PI, Hergeldzhieva TG (2009) Avastin use in high risk corneal transplantation. Graefes Arch Clin Exp Ophthalmol 247:1701–1706
Liu Y, Cao J, Renard RA et al (2006) Low-dose, subconjunctival administration of VEGF trap inhibits suture-induced corneal neovascularization and inflammation. In: Proceeding of the association for research in vision and ophthalmology (ARVO) annual meeting, Fort Lauderdale, FL, 30 April–4 May 2006. ARVO, Rockville
Breier G, Albrecht U, Sterrer S, Risau W (1992) Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 114:521–532
D’Amore PA (1994) Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci 35:3974–3979
Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT (2001) Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol 75:9828–9835
Maguire MG, Stark WJ, Gottsch JD, Stulting RD, Sugar A, Fink NE, Schwartz A (1994) Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative corneal transplantation studies research group. Ophthalmology 101:1536–1547
Williams KA, Lowe M, Bartlett C, Kelly TL, Coster DJ, All Contributors (2008) Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation 86:1720–1724
Ferrara N, Leung DW, Phillips HS (1991) Molecular characterization and distribution of vascular endothelial growth factor. In: Muller EE, MacLeod RB (eds) Neuroendocrine perspectives. Springer, New York p 127
Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP (1996) Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol 114:964–970
Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ (1996) A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci 37:1625–1632
Amano S, Rohan R, Kuroki M, Tolentino M, Adams AP (1998) Requirement for vascular endothelial growth factor in wound and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci 39:18–22
Gan L, Fagerholm P, Palmblad J (2004) Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand 82:557–563
Cursiefen C, Rummelt C, Kuchle M (2009) Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta 1 in human corneas with neovascularization. Cornea 19:526–533
Philipp W, Speicher L, Humpel C (2000) Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci 41:2514–2522
You IC, Kang IS, Lee SH, Yoon KC (2009) Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol 87:653–658
Zaki AA, Farid SF (2010) Subconjunctival bevacizumab for corneal neovascularization. Acta Ophthalmol 88:868–871
Yoeruek E, Ziemssen F, Henke-Fahle S, Tatar O, Tura A, Grisanti S, Bartz-Schmidt K, Szurman P; Tübingen Bevacizumab Study Group (2008) Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol 86:322–328
Chen WL, Lin CT, Lin NT, Tu IH, Li JW, Chow LP, Liu KR, Hu FR (2009) Subkonjoktival injection of bevacizumab (avastin)on corneal neovascularization in different rabbit models of corneal angiogenesis. Invest Ophthalmol Vis Sci 50:1659–1665
Hosseini H, Nejabat M, Mehryar M, Yazdehi T, Sedaghat A, Noori F (2007) Bevacizumab inhibits corneal neovascularization in an alkali burn induced model of corneal angiogenesis. Clin Exp Ophthalmol 35:745–748
Papathanassiou M, Theodossiadis PG, Liarakos VS, Rouvas A, Giamarellos-Bourboulis EJ, Vergados IA (2008) Inhibition of corneal neovascularization by subconunctival bevacizumab in an animal model. Am J Ophthalmol 145:424–431
Awadein A (2007) Subconjunctival bevacizumab for vascularized rejected corneal grafts. J Cataract Refract Surg 33:1991–1993
Erdurmus M, Totan Y (2007) Subconjuntival bevacizumab for corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 245:1577–1579
Bahar I, Kaiserman I, Mc Allum P, Rootman D, Slomovic A (2008) Subkonjunctival bevacizumab injection for corneal neovascularization. Cornea 27:142–147
Financial support
None.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acar, B.T., Halili, E. & Acar, S. The effect of different doses of subconjunctival bevacizumab injection on corneal neovascularization. Int Ophthalmol 33, 507–513 (2013). https://doi.org/10.1007/s10792-013-9732-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-013-9732-8