Abstract
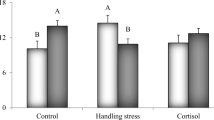
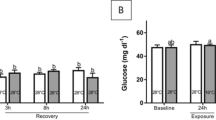
The year-round presence of ovigerous females of the parasite Caligus rogercresseyi in the fish farms of southern Chile results in a continuous source of the copepodid (infestive) stage of this louse. The short generation time in spring–summer could lead to high abundances of this copepodid, potentially leading to high infestation levels for fish. Knowing how heavy lice infestations affect Salmo salar can help determine how to time antiparasitic treatments so as to both minimize the treatment impact and reduce lice infestation levels for fish. This study aimed to describe the effects of high infestations of the copepodid stage of C. rogercresseyi on the physiology of S. salar. Two groups of S. salar were used: an infested group (75 copepodids per fish) and a control group (not infested). Sixty-five days after the first infestation, the infested fish group was re-infested at an infestation pressure of 200 copepodids per fish. Sampling was done prior to and following the second infestation, at 56 and 67 days (the latter 2 days following the second infestation). Several physiological variables were measured: cortisol (primary stress response) and glucose, proteins, amino acids, triglycerides, lactate, osmolality levels, and number and diameter of skin mucous cells (secondary stress responses). The plasma cortisol, glucose, and triglyceride levels were altered in the heavily infested fish, as was the diameter of skin mucous cells. These results suggest that heavy infestations of C. rogercresseyi lead to an acute stress response, metabolic reorganization, and increased mucus production in S. salar under heavy infestation conditions.



Similar content being viewed by others
Abbreviations
- Dpfi:
-
Days post first infestation
- Dpsi:
-
Days post second infestation
- IP:
-
Infestation pressure
References
Ansari RA, Rahman S, Kaur M, Anjum S, Raisuddin S (2011) In vivo cytogenetic and oxidative stress-inducing effects of cypermethrin in freshwater fish, Channa punctata Bloch. Ecotoxicol Environ Saf 74:150–156. doi:10.1016/j.ecoenv.2010.08.036
Barton B (2000) Salmonid fishes differ in their cortisol and glucose responses to handling and transport stress. N Am J Aquac. doi:10.1577/1548-8454(2000)062<0012:SFDITC>2.0.CO;2
Bowers JM, Mustafa A, Speare DJ, Conboy GA, Brimacombe M, Sims DE, Burka JF (2000) The physiological response of Atlantic salmon, Salmo salar L., to a single experimental challenge with sea lice, Lepeophtheirus salmonis. J Fish Dis 23:165–172. doi:10.1046/j.1365-2761.2000.00225.x
Bowers JM, Speare DJ, Burka JF (2002) The effects of hydrogen peroxide on the stress response of Atlantic Salmon (Salmo salar). J Vet Pharmacol Ther 25:311–313. doi:10.1046/j.1365-2885.2002.00413.x
Boxshall GA, Bravo S (2000) On the identity of the common Caligus (Copepoda: Siphonostomatoida: Caligidae) from salmonid netpen systems in southern Chile. Contrib Zool 69:137–146
Buchmann K, Bresciani J (1998) Microenvironment of Gyrodactylus derjavini on rainbow trout Oncorhynchus mykiss: association between mucous cell density in skin and site selection. Parasitol Res 84:17–24. doi:10.1007/s004360050350
Buchmann K, Bresciani J, Jappe C (2004) Effects of formalin treatment on epithelial structure and mucous cell densities in rainbow trout, Oncorhynchus mykiss (Walbaum), skin. J Fish Dis 27:99–104. doi:10.1111/j.1365-2761.2003.00519.x
Carvajal J, González L, George-Nascimento M (1998) Native sea lice (Copepoda: Caligidae) infestation of salmonids reared in netpen systems in southern Chile. Aquaculture 166:241–246. doi:10.1016/S0044-8486(98)00301-9
Costello MJ (2006) Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol 22:475–483. doi:10.1016/j.pt.2006.08.006
Costello MJ (2009) The global economic cost of sea lice to the salmonid farming industry. J Fish Dis 32:115–118. doi:10.1111/j.1365-2761.2008.01011.x
Ellis T, Yildiz HY, Lopez-Olmeda J, Spedicato MT, Tort L, Overli O, Martins CI (2012) Cortisol and finfish welfare. Fish Physiol Biochem 38:163–188. doi:10.1007/s10695-011-9568-y
Fast MD et al (2002) Susceptibility of rainbow trout Oncorhynchus mykiss, Atlantic salmon Salmo salar and coho salmon Oncorhynchus kisutch to experimental infection with sea lice Lepeophtheirus salmonis. Dis Aquat Org 52:57–68. doi:10.3354/dao052057
Fast MD, Muise DM, Easy RE, Ross NW, Johnson SC (2006) The effects of Lepeophtheirus salmonis infections on the stress response and immunological status of Atlantic salmon (Salmo salar). Fish Shellfish Immunol 21:228–241. doi:10.1016/j.fsi.2005.11.010
Fast MD, Hosoya S, Johnson SC, Afonso LOB (2008) Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol 24:194–204. doi:10.1016/j.fsi.2007.10.009
Foss A, Grimsbø E, Vikingstad E, Nortvedt R, Slinde E, Roth B (2012) Live chilling of Atlantic salmon: physiological response to handling and temperature decrease on welfare. Fish Physiol Biochem 38:565–571. doi:10.1007/s10695-011-9536-6
González L, Carvajal J (2003) Life cycle of Caligus rogercresseyi, (Copepoda: Caligidae) parasite of Chilean reared salmonids. Aquaculture 220:101–117. doi:10.1016/s0044-8486(02)00512-4
González MP, Marín SL, Vargas-Chacoff L (2015) Effects of Caligus rogercresseyi (Boxshall and Bravo, 2000) infestation on physiological response of host Salmo salar (Linnaeus 1758): establishing physiological thresholds. Aquaculture 438:47–54. doi:10.1016/j.aquaculture.2014.12.039
Heath AG (1995) Water pollution and fish physiology. CRC Press, Florida
Helgesen K, Bravo S, Sevatdal S, Mendoza J, Horsberg T (2014) Deltamethrin resistance in the sea louse Caligus rogercresseyi (Boxhall and Bravo) in Chile: bioassay results and usage data for antiparasitic agents with references to Norwegian conditions. J Fish Dis 37:877–890. doi:10.1111/jfd.12223
Hemre GI, Mommsen TP, Krogdahl Å (2002) Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquac Nutr 8:175–194. doi:10.1046/j.1365-2095.2002.00200.x
Heuch P, Nordhagen J, Schram T (2000) Egg production in the salmon louse (Lepeophtheirus salmonis (Krøyer)) in relation to origin and water temperature. Aquac Res 31:805–814. doi:10.1046/j.1365-2109.2000.00512.x
Holm H, Santi N, Kjøglum S, Perisic N, Skugor S, Evensen Ø (2015) Difference in skin immune responses to infection with salmon louse (Lepeophtheirus salmonis) in Atlantic salmon (Salmo salar L.) of families selected for resistance and susceptibility. Fish Shellfish Immunol 42:384–394. doi:10.1016/j.fsi.2014.10.038
Jung-Hoon J, Masroor F, Ju-Chan K (2005) Responses of cypermethrin-induced stress in haematological parameters of Korean rockfish, Sebastes schlegeli (Hilgendorf). Aquac Res 36:898–905. doi:10.1111/j.1365-2109.2005.01299.x
Krasnov A, Skugor S, Todorcevic M, Glover KA, Nilsen F (2012) Gene expression in Atlantic salmon skin in response to infection with the parasitic copepod Lepeophtheirus salmonis, cortisol implant, and their combination. BMC Genom 13:130. doi:10.1186/1471-2164-13-130
Krkošek M, Bateman A, Proboszcz S, Orr C (2010) Dynamics of outbreak and control of salmon lice on two salmon farms in the Broughton Archipelago, British Columbia. Aquac Environ Interact 1:137–146. doi:10.3354/aei00014
Lower N, Moore A (2003) Exposure to insecticides inhibits embryo development and emergence in Atlantic salmon (Salmo salar L.). Fish Physiol Biochem 28:431–432. doi:10.1023/B:FISH.0000030617.74673.92
Marín SL, Martin R, Lewis R (2015) Effects of Caligus rogercresseyi (Boxshall & Bravo 2000) chalimus stage condition (dead, moribund, live) on the estimates of Cypermethrin BETAMAX® efficacy. Aquac Res 46:30–36. doi:10.1111/are.12460
Meyer C, Ganter M, Korting W, Steinhagen D (2002) Effects of a parasite-induced nephritis on osmoregulation in the common carp Cyprinus carpio. Dis Aquat Org 50:127–135
Moberg GP, Mench JA (2000) The biology of animal stress: basic principles and implications for animal welfare. CABI Publishing, New York
Molinari N, Daurés JP, Durand JF (2001) Regression splines for threshold selection in survival data analysis. Stat Med 20:237–247. doi:10.1002/1097-0258(20010130)20:2<237:AID-SIM654>3.0.CO;2-I
Molinet C et al (2011) Population dynamic of early stages of Caligus rogercresseyi in an embayment used for intensive salmon farms in Chilean inland seas. Aquaculture 312:62–71. doi:10.1016/j.aquaculture.2010.12.010
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268. doi:10.1023/A:1008924418720
Nolan DT, Reilly P, Wendelaar Bonga SE (1999) Infection with low numbers of the sea louse Lepeophtheirus salmonis induces stress-related effects in postsmolt Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 56:947–959. doi:10.1139/f99-021
Olafsdottir SH, Buchmann K (2004) Dexamethasone treatment affects skin mucous cell density in Gyrodactylus derjavini infected Salmo salar. J Helminthol 78:87–90. doi:10.1079/JOH2003206
Olsvik PA, Ørnsrud R, Lunestad BT, Steine N, Fredriksen BN (2014) Transcriptional responses in Atlantic salmon (Salmo salar) exposed to deltamethrin, alone or in combination with azamethiphos. Comp Biochem Phys C 162:23–33. doi:10.1016/j.cbpc.2014.03.005
Pankhurst N, Ludke S, King H, Peter R (2008) The relationship between acute stress, food intake, endocrine status and life history stage in juvenile farmed Atlantic salmon, Salmo salar. Aquaculture 275:311–318. doi:10.1016/j.aquaculture.2008.01.001
Pittman K et al (2013) Body site matters: an evaluation and application of a novel histological methodology on the quantification of mucous cells in the skin of Atlantic salmon, Salmo salar L. J Fish Dis 36:115–127. doi:10.1111/jfd.12002
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Redding JM, Patiño R, Schreck CB (1984) Clearance of corticosteroids in yearling coho salmon, Oncorhynchus kisutch, in fresh water and seawater and after stress. Gen Comp Endocrinol 54:433–443. doi:10.1016/0016-6480(84)90159-X
Russ JC, DeHoff RT (2000) Practical stereology, vol 1. Kluwer Academic/Plenum, New York
Sandodden R, Finstad B, Iversen M (2001) Transport stress in Atlantic salmon (Salmo salar L.): anaesthesia and recovery. Aquac Res 32:87–90. doi:10.1046/j.1365-2109.2001.00533.x
SERNAPESCA (2014) Aprueba Tratamiento coordinado de invierno contra Caligidosis. Ministerio de Economía Fomento y Turismo, Valparaíso
Shea E, Vecchione M (2002) Quantification of ontogenetic discontinuities in three species of oegopsid squids using model II piecewise linear regression. Mar Biol 140:971–979. doi:10.1007/s00227-001-0772-7
Skjervold PO, Fjæra SO, Østby PB, Einen O (2001) Live-chilling and crowding stress before slaughter of Atlantic salmon (Salmo salar). Aquaculture 192:265–280. doi:10.1016/S0044-8486(00)00447-6
Sutherland B, Koczka K, Yasuike M, Jantzen S, Yazawa R, Koop B, Jones S (2014) Comparative transcriptomics of Atlantic Salmo salar, chum Oncorhynchus keta and pink salmon O. gorbuscha during infections with salmon lice Lepeophtheirus salmonis. BMC Genom 15(1):200. doi:10.1186/1471-2164-15-200
Treasurer JW, Bravo S (2011) The spatial distribution patterns of Caligus rogercresseyi and C. elongatus on Atlantic salmon hosts (Salmo salar). Aquaculture 320:154–158. doi:10.1016/j.aquaculture.2011.03.032
Tseng Y-C, Hwang P-P (2008) Some insights into energy metabolism for osmoregulation in fish. Comp Biochem Phys C 148:419–429. doi:10.1016/j.cbpc.2008.04.009
Tucker CS, Sommerville C, Wootten R (2000) The effect of temperature and salinity on the settlement and survival of copepodids of Lepeophtheirus salmonis (Krøyer, 1837) on Atlantic salmon, Salmo salar L. J Fish Dis 23:309–320. doi:10.1046/j.1365-2761.2000.00219.x
Ucán-Marín F, Ernst W, O’Dor RK, Sherry J (2012) Effects of food borne ivermectin on juvenile Atlantic salmon (Salmo salar L.): Survival, growth, behavior, and physiology. Aquaculture 334–337:169–175. doi:10.1016/j.aquaculture.2011.12.036
Vargas-Chacoff L, Arjona FJ, Polakof S, del Rio MP, Soengas JL, Mancera JM (2009) Interactive effects of environmental salinity and temperature on metabolic responses of gilthead sea bream Sparus aurata. Comp Biochem Physiol Part A Mol Integr Physiol 154:417–424. doi:10.1016/j.cbpa.2009.07.015
Vargas-Chacoff L et al (2011) Growth performance, osmoregulatory and metabolic modifications in red porgy fry, Pagrus pagrus, under different environmental salinities and stocking densities. Aquac Res 42:1269–1278. doi:10.1111/j.1365-2109.2010.02715.x
Vargas-Chacoff L et al (2014a) Combined effects of high stocking density and Piscirickettsia salmonis treatment on the immune system, metabolism and osmoregulatory responses of the sub-Antarctic Notothenioid fish Eleginops maclovinus. Fish Shellfish Immunol 40:424–434. doi:10.1016/j.fsi.2014.07.024
Vargas-Chacoff L, Moneva F, Oyarzún R, Martínez D, Muñoz JLP, Bertrán C, Mancera JM (2014b) Environmental salinity-modified osmoregulatory response in the sub-Antarctic notothenioid fish Eleginops maclovinus. Polar Biol 37:1235–1245. doi:10.1007/s00300-014-1515-9
Vargas-Chacoff L et al (2014c) Stocking density and Piscirickettsia salmonis infection effect on Patagonian blennie (Eleginops maclovinus, Cuvier 1830) skeletal muscle intermediate metabolism. Fish Physiol Biochem 40:1683–1691. doi:10.1007/s10695-014-9959-y
Vargas-Chacoff L, Ruiz-Jarabo I, Páscoa I, Gonçalves O, Mancera JM (2014d) Yearly growth and metabolic changes in earthen pond-cultured meagre. Sci Mar 78:193–202. doi:10.3989/scimar.03965.06B
Vatsos I, Kotzamanis Y, Henry M, Angelidis P, Alexis M (2010) Monitoring stress in fish by applying image analysis to their skin mucous cells. Eur J Histochem EJH 54:107–111. doi:10.4081/ejh.2010.e22
Vijayan MM, Ballantyne JS, Leatherland JF (1990) High stocking density alters the energy metabolism of brook charr, Salvelinus fontinalis. Aquaculture 88:371–381. doi:10.1016/0044-8486(90)90162-G
Wedemeyer G (1996) Physiology of fish in intensive culture systems, 1st edn. Springer, Dordrecht
Wells A et al (2006) Physiological effects of simultaneous, abrupt seawater entry and sea lice (Lepeophtheirus salmonis) infestation of wild, sea-run brown trout (Salmo trutta) smolts. Can J Fish Aquat Sci 63:2809–2821. doi:10.1139/f06-160
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Wood CM (1991) Acid–base and ion balance, metabolism, and their interactions, after exhaustive exercise in fish. J Exp Biol 160:285–308
Acknowledgments
The authors want to thank the Instituto de Fomento Pesquero for supporting this study, Marine Harvest Chile for providing experimental fish, and FONDECYT 1110235. M.P. González is funded by Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) with a National Doctoral Scholarship and wants to thank the Fish Physiology Laboratory and LINTEC Laboratory of Universidad Austral de Chile.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González, M.P., Vargas-Chacoff, L. & Marín, S.L. Stress response of Salmo salar (Linnaeus 1758) when heavily infested by Caligus rogercresseyi (Boxshall & Bravo 2000) copepodids. Fish Physiol Biochem 42, 263–274 (2016). https://doi.org/10.1007/s10695-015-0134-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0134-x