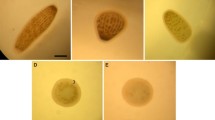
Abstract
We have investigated the chronic effects of low concentrations of lead (Pb) on oxidative damage and DNA damage in testes of the frog Rana nigromaculata. Sixty adult male frogs were randomly divided into six groups of ten. Based on the levels of the Integrated Wastewater Discharge Standard (GB 8978-1996) of China, five groups (II–VI) were treated by epidermal absorption with a PbNO3 solution at concentrations of 0.1, 0.2, 0.4, 0.8, 1.6 mg/l, respectively. The first group (I), which served as a control, was treated with distilled water only. Thirty days after treatment, all frogs were sacrificed and the testis tissues removed for the measurement of malondialdehyde (MDA) and glutathione (GSH) levels. DNA damage, including indicators of damage rate, DNA tail length (TL), and DNA tail moment (TM), was also analyzed by comet assays. Our data suggest that MDA levels in all treatment groups and GSH levels in the 0.2–1.6 mg/l Pb groups increased significantly relative to the controls (P < 0.01). Treatment with Pb at concentrations >0.4 mg/l also increased DNA damage rate and TM, while TL increased when the Pb level was >0.2 mg/l (P < 0.01 for DNA damage rate and TM, P < 0.05 for TL). Positive correlations were also found between DNA damage levels in the testes and MDA levels (r = 0.796 for DNA damage rate, r = 0.811 for TL, r = 0.796 for TM; P < 0.01 for all) as well between MDA and GSH levels (r = 0.455, P < 0.05) in the testes. Results from MDA measurements indicated that Pb-induced DNA damage in the testes of R. nigromaculata was possibly due to oxidative damage. Taken together, we conclude that Pb can induce male reproductive toxicity in R. nigromaculata.
Similar content being viewed by others
References
Ahamed M, Verma S, Kumar A, Siddiqui MKJ (2005) Environmental exposure to lead and its correlation with biochemical indices in children. Sci Total Environ 346:48–55. doi:10.1016/j.scitotenv.2004.12.019
Beebee TJC, Griffiths RA (2005) The amphibian decline crisis: a watershed for for conservation biology? Biol Conserv 125:271–285. doi:10.1016/j.biocon.2005.04.009
Berrill M, Bertram S, Pauli B (1997) Effects of pesticides on amphibian embryos and tadpoles. In: Green DM (ed) Amphibians in decline: Canadian studies of a global problem. Society for the Study of Amphibians and Reptiles, St. Louis, pp 57–63
Blaustein AR, Hoffman PD, Hokit DG, Kiesecker JM, Walls SC, Hays JB (1994) UV repair and resistance to solar UV-B in amphibian eggs: a link to population declines. Proc Natl Acad Sci USA 91:1791–1795. doi:10.1073/pnas.91.5.1791
Chiesa ME, Rosenberg CE, Fink NE, Salibián A (2006) Serum protein profile and blood cell counts in adult toads Bufo Arenarum (Amphibia: Anura: Bufonidae): effects of sublethal lead acetate. Arch Environ Contam Toxicol 50:384–391. doi:10.1007/s00244-004-0252-4
Corn PS (2000) Amphibian declines: review of some current hypotheses. In: Sparling DW, Linder G, Bioshop CA (eds) Ecotoxicology of amphibians and reptiles. SETAC Press, Pensacola, pp 633–696
Danadevi K, Rozati R, Banu BS, Rao PH, Grover P (2003) DNA damage in workers exposed to lead using comet assay. Toxicology 187:183–189. doi:10.1016/S0300-483X(03)00054-4
Daszak P, Cunningham AA, Hyatt AD (2003) Infectious disease and amphibian population declines. Divers Distrib 9:141–150. doi:10.1046/j.1472-4642.2003.00016.x
Davidson C (2004) Declining downwind: amphibian population declines in California and historical pesticide use. Ecol Appl 14:1892–1902. doi:10.1890/03-5224
García-García G, Nandini S, Sarma SSS (2006) Turbidity mitigates lead toxicity to cladocerans (Cladocera). Ecotoxicology 15:425–436. doi:10.1007/s10646-006-0064-6
Guo J (2005) The study on DNA damage of freshwater crab (Sinopotamon yangteskiense) resulting from cadmium and lead (in Chinese). MSc thesis. Shangxi University, Shangxi
Gurer H, Ozgunes H, Neal R, Spitz DR, Ercal N (1998) Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead exposed rats. Toxicology 128:181–189. doi:10.1016/S0300-483X(98)00074-2
Hels T, Buchwald E (2001) The effect of road kills on amphibian populations. Biol Conserv 99:331–340. doi:10.1016/S0006-3207(00)00215-9
Jin YP, Liao YJ, Lu CW, Li GX, Yu F, Zhi XP et al (2006) Health effects in children aged 3–6 years induced by environmental lead exposure. Ecotoxicol Environ Saf 63:313–317. doi:10.1016/j.ecoenv.2005.05.011
Kats LB, Ferrer RP (2003) Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers Distrib 9:99–100. doi:10.1046/j.1472-4642.2003.00013.x
Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian declines. Nature 410:681–684. doi:10.1038/35070552
Lips KR (1999) Mass mortality and population declines of anurans at an upland site in western Panama. Conserv Biol 13:117–125. doi:10.1046/j.1523-1739.1999.97185.x
Liu HG, Wang Y, Lian LJ, Xu LH (2006a) Tributyltin induces DNA damage as well as oxidative damage in rats. Environ Toxicol 21:166–171. doi:10.1002/tox.20170
Liu Y, Zhang YM, Liu JH, Huang DJ (2006b) The role of reactive oxygen species in the herbicide acetochlor-induced DNA damage on Bufo raddei tadpole liver. Aquat Toxicol 78:21–26. doi:10.1016/j.aquatox.2006.01.016
Lu AL, Li X, Gu Y, Wright PM, Chang DY (2001) Repair of oxidative DNA damage: mechanisms and functions. Cell Biochem Biophys 35:141–170. doi:10.1385/CBB:35:2:141
Middleton EM, Herman JR, Celarier EA, Wilkinson JW, Carey C, Rusin RJ (2001) Evaluating ultraviolet radiation exposure with satellite data at sites of amphibian declines in central and South America. Conserv Biol 15:914–929. doi:10.1046/j.1523-1739.2001.015004914.x
Morgan LA, Buttemer X (1996) Predation by the non-native fish Gambusia holbrooki on small Litoria aurea and L. dentata tadpoles. Aust Zool 30:143–149
Nystrom P, Hansson J, Mansson J, Sundstedt M, Reslow C, Brostrom A (2007) A documented amphibian decline over 40 years: possible causes and implications for species recovery. Biol Conserv 138:399–411. doi:10.1016/j.biocon.2007.05.007
Oakes KD, Sibley PK, Martin JW, Maclean DD, Solomon KR, Mabury SA et al (2005) Short-term exposures of fish to perfluorooctane sulfonate: acute effects of fatty acyl-CoA oxidase activity, oxidative stress, and circulating sex steroids. Environ Toxicol Chem 24:1172–1181. doi:10.1897/04-419.1
Olive PL, Banath JP, Durand RE (1990) Haterogeneity in radiation induced DNA damage and repair in tumor and normal cells measured using the “comet assay”. Radiat Res 122:84–90. doi:10.2307/3577587
Panadimitriou E, Loumbourdis NS (2002) Exposure of the Rana ridibumda to copper: impact on two biomarkers, lipid peroxidation, and glutathione. Bull Environ Contam Toxicol 69:885–891. doi:10.1007/s00128-002-0142-2
Pande M, Flora SJS (2002) Lead induced oxidative damage and its response to combined administration of α-lipoic acid and succimers in rats. Toxicology 177:187–196. doi:10.1016/S0300-483X(02)00223-8
Pounds JA, Fogden MPL, Campbell JH (1999) Biological response to climate change on a tropical mountain. Nature 398:611–615. doi:10.1038/19297
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischmann DL et al (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786. doi:10.1126/science.1103538
Vogiatzis AK, Loumbourdis NS (1998) Cadmium accumulation in liver and kidneys and hepatic metallothionein and glutathione levels in Rana ridibumda, after exposure to CdCl2. Arch Environ Contam Toxicol 34:64–68. doi:10.1007/s002449900286
Yang JM, Arnush M, Chen QY, Wu XD, Pang B, Jiang XZ (2003) Cadmium-induced damage to primary cultures of rat Leydig cells. Reprod Toxicol 17:553–560. doi:10.1016/S0890-6238(03)00100-X
Yuan J, Liu H, Zhou LH, Zou YL, Lu WQ (2006) Oxidative stress and DNA damage induced by a drinking-water chlorination disinfection byproduct 3-chloro-4-(dichloromethyl)-5- hydroxy- 2(5H)-furanone (MX) in mice. Mutat Res 609:129–136
Zhang YM, Huang DJ, Zhao DQ (2007) Long-term toxicity effects of cadmium and lead on Bufo raddei tadpoles. Bull Environ Contam Toxicol 79:178–183. doi:10.1007/s00128-007-9152-4
Zhang YM, Wang YJ, Yu RL, Zhang S, Wu ZB (2008) Effects of heavy metals Cd2+, Pb2+, Zn2+ on DNA damage of loach Misgurnus anguillicaudatus. Front Biol China 3:50–54. doi:10.1007/s11515-008-0012-3
Acknowledgments
This research was supported by the Natural Science Foundation of Zhejiang Province in China (Grant No. 302056). We thank Ms. Guo-Ying Wu and Mr. Ji Qiu for the assistance in the frog breeding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, MZ., Jia, XY. Low levels of lead exposure induce oxidative damage and DNA damage in the testes of the frog Rana nigromaculata . Ecotoxicology 18, 94–99 (2009). https://doi.org/10.1007/s10646-008-0262-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0262-5