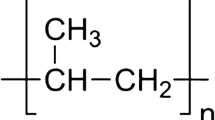Abstract
The physical structure and properties of ethylcellulose (EC) powders of different molecular weights were examined. A molecular weight in the range of 20–144 kDa with a large polydispersity was determined. EC thermal analysis revealed a glass transition at ~130 °C and a melting temperature at ~180 °C. Glass transition temperatures increased with polymer molecular weight. Wide angle (WAXS) analysis detected an amorphous broad peak at q = 1.5 Å−1 and a distinct Bragg’s peak at 12.6 Å, which seems to be related to a supramolecular ordered structure of the polymer. These observations were confirmed using high temperature powder X-ray diffraction analysis where the crystalline peak disappeared above the melting temperature of the polymer. Ultra-small angle (USAXS) results were fitted to the Bouacage fractal unified model and fractals with an average size of 100–600 nm with a relatively smooth surface were predicted. This prediction was confirmed by transmission electron microscopy (TEM) images. According to our results, the EC polymer has a semi-crystalline structure, with crystalline domains within an amorphous background.








Similar content being viewed by others
References
Agarwal V, Huber GW, Conner WC Jr, Auerbach SM (2011) Simulating infrared spectra and hydrogen bonding in cellulose Iβ at elevated temperatures. J Chem Phys 135:1–13
Aiache JM, Gauthier P, Aiache S (1992) New gelification method for vegetable oils I: cosmetic application. Int J Cosmetic Sci 14:228–234
Atalla RH, Isogai A (1998) Recent developments in spectroscopic and chemical characterization of cellulose. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility, 2nd edn. Marcel Dekker, New York, pp 123–157
Beaucage G (1995) Approximations leading to a unified exponential/power-law approach to small-angle scattering. J Appl Cryst 28:717–728
Beaucage G (1996) Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. J Appl Cryst 29:134–146
Cavalcanti OA, Petenuc B, Bedin AC, Pineda EAG, Hechenleitner AAW (2004) Characterisation of ethylcellulose films containing natural polysaccharides by thermal analysis and FTIR spectroscopy. Acta Farm Bonaerense 23:53–57
Cousins SK, Brown RM (1995) Cellulose I microfibril assembly: computational molecular mechanics energy analysis favours bonding by van der Waals forces as initial step in crystalization. Polymer 36:3885–3888
Crowley MM, Schroeder B, Fredersdorf A, Obara S, Talarico M, Kucera S, McGinity JW (2004) Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int J Pharm 269:509–522
Duarte ARC, Gordillo MD, Cardoso MM, Simplicio AL, Duarte CMM (2006) Preparation of ethyl cellulose/methyl cellulose blends by supercritical antisolvent precipitation. Int J Pharm 311:50–54
Ethylcellulose polymers technical handbook (2005). Dow Chemical Company, http://www.dow.com/dowwolff/en/pdfs/192-00818.pdf
Flory PJ, Vrij A (1963) Melting points of linear-chain homologs, the normal paraffin hydrocarbons. J Am Chem Soc 85:3548–3553
Follonier N, Doelker E, Cole ET (1994) Evaluation of hot-melt extrusion as a new technique for the production of polymer-based pellets for sustained release capsules containing high loading of freely soluble drugs. Drug Dev Ind Pharm 20:1323–1339
Fox TG, Flory PJ (1950) Second order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys 21:581–591
Freelon B, Sutharb K, Ilavsky J (2013) A multi-length-scale USAXS/SAXS facility: 10–50 keV small-angle X-ray scattering instrument. J Appl Cryst 46:1508–1512
French AD (2012) Combining computational chemistry and crystallography for a better understanding of the structure of cellulose. Adv Cacbohyd Chem Biochem 67:19–93
Gopalan M, Mandelkern L (1967) The effect of crystallization temperature and molecular weight on the melting temperature of linear polyethylene. J Phys Chem 71:3833–3841
Hughes NE, Marangoni AG, Wright AJ, Rogers MA, Rush JWE (2009) Potential food applications of edible oil organogels. Food Sci Tech 20:470–480
Ilavsky J (2012) Nika: software for two-dimensional data reduction. J Appl Cryst 45:324–328
Ilavsky J, Jemian PR (2009) Irena: tool suite for modeling and analysis of small-angle scattering. J Appl Cryst 42:347–353
Ilavsky J, Jemian PR, Allen AJ, Zhang F, Levine LE, Long GG (2009) Ultra-small-angle X-ray scattering at the advanced photon source. J Appl Cryst 42:469–479
Ilavsky J, Allen AJ, Levine LE, Zhang F, Jemianc PR, Longa GG (2012) High-energy ultra-small-angle X-ray scattering instrument at the advanced photon source. J Appl Cryst 45:1318–1320
Isogai A, Atalla RH (1998) Dissolution of cellulose in aqueous NaOH solutions. Cellulose 5:309–319
Jeffries R (1968) Preparation and properties of films and fibers of disordered cellulose. J Appl Polym Sci 12:425–445
Jullien R (1987) Aggregation phenomena and fractal aggregates. Contemp Phys 28:477–493
Knill CJ, Kennedy JF (1998) Cellulosic biomass-derived products. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility. Marcel Dekker, New York, pp 937–956
Koch W (1937) Properties and uses of ethylcellulose. Ind Ing Chem 29:687–690
Kondo T (1998) Hydrogen bonds in cellulose and cellulose derivatives. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility, 2nd edn. Marcel Dekker, New York, pp 69–98
Kondo T, Sawatari C (1996) A Fourier transform infra-red spectroscopic analysis of the character of hydrogen bonds in amorphous cellulose. Polymer 37:393–399
Maki R, Suihko E, Korhonen O, Pitkanen H, Niemi R, Lehtonen M, Ketolainen J (2006) Controlled release of saccharides from matrix tablets. Eur J Pharm Biopharm 62:163–170
Meakin P, Jullien R (1985) Structural readjustment effects in cluster–cluster aggregation. J Phys France 46:1543–1552
Moore WR, Brown AM (1959) Viscosity-temperature relationships for dilute solutions of cellulose derivatives II. Intrinsic viscosities of ethyl cellulose. J Colloid Sci 14:343–353
Morris ER, Cutler AN, Ross-Murphy SB, Rees DA (1981) Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohyd Polym 1:5–21
Nelson ML, O’Conner RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part I. Spectra of lattice types I, 11, I11 and of amorphous cellulose. J Appl Polym Sci 8:1311–1324
O’sullivan AC (1997) Cellulose: the structure slowly unravels. Cellulose 4:173–207
Overney RM, Buenviaje C, Luginbuhl R, Dinelli F (2000) Glass and structural transitions measured at polymer surfaces on the nanoscale. J Therm Anal Cal 59:205–225
Perez S, Samain D (2010) Structure and engineering of celluloses. Adv Cacbohyd Chem Biochem 64:26–116
Rekhi GS, Jambhekar SS (1995) Ethylcellulose: a polymer review. Drud Dev Ind Pharm 21:61–77
Repka MA et al (2007) Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm 33:1043–1057
Roos Y, Karel M (1991) Water and molecular weight effects on glass transitions in amorphous carbohydrates and carbohydrate solutions. J Food Sci 56:1676–1681
Roudaut G, Simatos D, Champion D, Contreras-Lopez E, Meste ML (2004) Molecular mobility around the glass transition temperature: a mini review. Innov Food Sci Emerg Tech 5:127–134
Rowe RC, Kotaras AD, White EFT (1984) An evaluation of the plasticizing efficiency of the dialkyl phthalates in ethyl cellulose films using the torsional braid pendulum. Int J Pharm 22:57–62
Roy D, Semsarilar M, Guthrie JT, Perrier S (2009) Cellulose modification by polymer grafting: a review. Chem Soc Rev 38:2046–2064
Sakellariou P, Rowe RC, White EFT (1985) The thermo mechanical properties and glass transition temperatures of some cellulose derivatives used in film coating. Int J Pharm 27:267–277
Sandford PA, Cottrell IW, Pettitt DJ (1984) Microbial polysaccharides: new products and their commercial applications. Pure Appl Chem 56:879–892
Schaefer DW, Hurd AJ (1990) Growth and structure of combustion aerosols fumed silica. Aerosol Sci Tech 12:876–890
Sinha SK, Freltoft T, Kjems J (1984) Observation of power law correlations in silica-particle aggregates by small-angle neutron scattering. In: Family F, Landau DP (eds) Kinetics of aggregation and gelation. North-Holland Physics Publishing, Amsterdam, pp 87–90
Skillas G et al (2002) Relation of the fractal structure of organic pigments to their performance. J Appl Phys 91:6120–6124
Soares JP, Santos JE, Chierice GO, Cavalheiro ETG (2004) Thermal behavior of alginic acid and its sodium salt. Eclet Quim 29:57–63
Tarvainen M et al (2003) Enhanced film-forming properties for ethyl cellulose and starch acetate using n-alkenyl succinic anhydrides as novel plasticizers. Eur J Pharm Sci 19:363–371
Torres FE, Russel WB, Schowalter WR (1991) Simulations of coagulation in viscous flows. J Colloid Interface Sci 145:51–73
Wada M (2002) Lateral thermal expansion of cellulose I and I III Polymorphs. J Polym Sci B: Polym Phys 40:1095–1102
Wada M, Hori R, Kim U-J, Sasaki S (2010) X-ray diffraction study on the thermal expansion behavior of cellulose Ib and its high-temperature phase. Polym Deg Stab 95:1330–1334
Ward TC (1981) Molecular weight and molecular weight distributions in synthetic polymers. J Chem Edu 58:867–879
Witten TA, Sander LM (1983) Diffusion-limited aggregation. Phys Rev B 27:5686–5697
Yu DG, Yang XL, Huang WD, Liu J, Wang YG, Xu H (2006) Tablets with material gradients fabricated by three-dimensional printing. J Pharm Sci 96:2446–2456
Zetzl AK, Marangoni AG, Barbut S (2012) Mechanical properties of ethylcellulose oleo gels and their potential for saturated fat reduction in frankfurters. Food Funct 3:327–337
Zuluaga R, Putaux JL, Cruz J, Vélez J, Mondragon I, Gañán P (2009) Cellulose microfibrils from banana rachis: effect of alkaline treatments on structural and morphological features. Carbohyd Polym 76:51–59
Acknowledgments
Research supported by the Ontario Ministry of Agriculture and Food (OMAF) and the Natural Sciences and Engineering Research Council of Canada (NSERC). We acknowledge the technical assistance of Fernanda Peyronel for setting up experiments and data analysis. The author wish to thank Dr. Jan Ilavsky from the APS sector 15ID-D USAXS/SAXS facility for his help conducting both SAXS and USAXS experiments. ChemMatCARS Sector 15 is principally supported by the National Science Foundation/Department of Energy under grant number NSF/CHE-0822838. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davidovich-Pinhas, M., Barbut, S. & Marangoni, A.G. Physical structure and thermal behavior of ethylcellulose. Cellulose 21, 3243–3255 (2014). https://doi.org/10.1007/s10570-014-0377-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0377-1