Abstract
A new aminophosphine palladium(0) complex supported on ZrO2 nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) was successfully synthesized and characterized using FT-IR, XRD, XPS, SEM, TEM, EDS, TGA and ICP techniques. Characterization results revealed that the synthesized catalyst had tetragonal and monoclinic structure with spherical morphology. The prepared nanocatalyst was showed excellent reactivity in the Suzuki–Miyaura and Heck–Mizoroki cross-coupling reactions. Moreover, this nanocatalyst can be easily recovered and reused for at least six cycles without deterioration in catalytic activity.
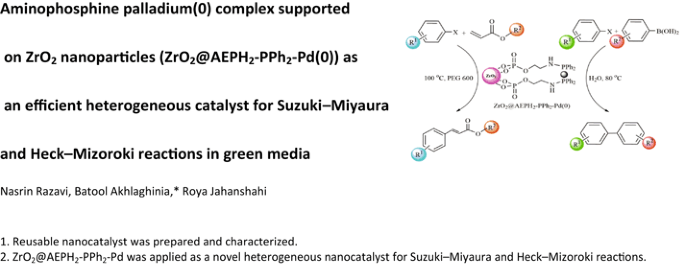
Graphical Abstract










Similar content being viewed by others
References
Johannes GV (2001) Can J Chem 79:1086–1092
Leroux F (2004) ChemBioChem 5:644–649
Lightowler S, Hird M (2005) Chem Mater 17:5538–5549
Kozlowski MC, Morgan BJ, Linton EC (2009) Chem Soc Rev 38:3193–3207
Ogata A, Furukawa C, Sakurai K, Iba H, Kitade Y, Ueno Y (2010) Bioorg Med Chem Lett 20:7299–7302
Maitlis PM, Haynes A (2006) Metal-catalysis in industrial organic processes. RSC Publishing, Cambridge
Tappe FMJ, Trepohl VT, Oestreich M (2010) Synthesis 2010:3037–3062
Hiyama T, Diederich F, Stang PJ (1998) Metal-catalyzed cross-coupling reactions. Wiley-VCH, Weinheim
Ojima I (2000) Catalytic asymmetric synthesis. Wiley-VCH, New York
Miyaura N, Yamada K, Suzuki A (1979) Tetrahedron Lett 20:3437–3440
Heck RF, Nolley JP (1972) J Org Chem 37:2320–2322
Dieck HA, Heck RF (1974) J Am Chem Soc 96:1133–1136
Larhed M, Hallberg A, Negishi E (2002) Handbook of organopalladium chemistry for organic synthesis. Wiley, New York
Beletskaya IP, Cheprakov AV (2000) Chem Rev 100:3009–3066
Tao L, Xie Y, Deng C, Li J (2009) Chin J Chem 27:1365–1373
Chanjuan X, Yongwei W, Xiaoyu Y (2008) J Organomet Chem 693:3842–3846
Koji S, Ryouta T, Tsukasa N, Hisashi F (2008) Angew Chem Int Ed 47:6917–6919
Yan R, Xu JX, Zhang YY, Wang D, Zhang MC, Zhang WQ (2012) Chem Eng J 200:559–568
Al-Hashimi M, Sullivan AC, Wilson JRH (2007) J Mol Catal A Chem 273:298–302
Bohrsch V, Hackenberger CPR (2010) ChemCatChem 2:243–245
Yang F, Li YF, Liu T, Xu K, Zhang LQ, Xu CM, Gao JS (2013) Chem Eng J 226:52–58
Moussa S, Siamaki AR, Gupton BF, Samy El-Shall M (2012) ACS Catal 2:145–154
Rana S, Maddila S, Yalagala K, Jonnalagadda SB (2015) Appl Catal A 505:539–547
Okumura K, Tomiyama T, Okuda S, Yoshida H, Niwa M (2010) J Catal 273:156–166
Shylesh S, Wang L, Thiel WR (2010) Adv Synth Catal 352:425–432
Beygzadeh M, Alizadeh A, Khodaei MM, Kordestani D (2013) Catal Commun 32:86–91
Zhang YY, Zhang MC, Zhang X, Wang XH, Zhang WQ (2013) Chem Eng J 215:96–104
Proch S, Mei Y, Villanueva JMR, Lu Y, Karpov A, Ballauff M, Kempe R (2008) Adv Synth Catal 350:493–500
Ramarao C, Ley SV, Smith SC, Shirley IM, DeAleida N (2002) Chem Commun 10:1132–1133
Ahn JM, Wentworth P, Janda KD (2003) Chem Commun 4:490–491
Uozumi Y, Yamada YMA, Beppu T, Fukuyama N, Ueno M, Kitamori T (2006) J Am Chem Soc 128:15994–15995
Biffis A, Zecca M, Basato M (2001) J Mol Catal A Chem 173:249–274
Astruc D, Lu F, Aranzaes JR (2005) Angew Chem Int Ed 44:7852–7872
Stevens PD, Fan JD, Gardinmalla HMR, Yen M (2005) Org Lett 7:2085–2088
Barder TE, Walker SD, Martinelli JR, Buchwald SL (2005) J Am Chem Soc 127:4685–4696
Falvello LR, Ginés JC, Carbó JJ, Lledos A, Navarro R, Soler T, Urriolabeitia EP (2006) Inorg Chem 45:6803–6815
Yan X, Liu Y, Xi C (2008) Appl Organomet Chem 22:341–345
Phan NTS, VanderSluys M, Jones CW (2006) Adv Synth Catal 348:609–679
Trzeciak AM, Ziółkowski J (2005) J Coord Chem Rev 249:2308–2322
Trzeciak AM, Ziółkowski J (2007) J Coord Chem Rev 251:1281–1293
Liu Sh, Han MY (2010) Chem Asian J 5:36–45
Lu J, Zang JB, Shan SX, Huang H, Wang YH (2008) Nano Lett 8:4070–4074
Luo X, Morrin A, Killard AJ, Smyth MR (2006) Electroanalysis 18:319–326
Steiner SA, Baumann ThF, Bayer BC, Blume R, Worsley MA, MoberlyChan WJ, Shaw EL, Schlogl R, Hart AJ, Hofmann S, Wardle BL (2009) J Am Chem Soc 131:12144–12154
Gawande MB, Shelke SN, Branco PS, Rathi A, Pandey RK (2012) Appl Organomet Chem 26:395–400
Gawande MB, Branco PS, Parghi K, Shrikhande JJ, Pandey RK, Ghumman CAA, Bundaleski N, Teodoro OMND, Jayaram RV (2011) Catal Sci Technol 1:1653–1664
Gawande MB, Rathi AK, Branco PS, Potewar TM, Velhinho A, Nogueira ID, Tolstogouzov A, Ghumman CAA, Teodoro OMND (2013) RSC Adv 3:3611–3617
Liu H, Cheung P, Iglesia E (2003) J Phys Chem B 107:4118–4127
Xu X, Wang X (2009) Nano Res 2:891–902
Nakka L, Molinari JE, Wachs IE (2009) J Am Chem Soc 131:15544–15554
Tomishig K, Ikeda Y, Sakaihori T, Fujimoto K (2000) J Catal 192:355–362
Li W, Huang H, Li H, Zhang W, Liu H (2008) Langmuir 24:8358–8366
Wang R, Crozier PA, Sharma R, Adams JB (2008) Nano Lett 8:962–967
Monopoli A, Nacci A, Calo V, Ciminale F, Cotugno P, Mangone A, Giannossa LC, Azzone P, Cioffi N (2010) Molecules 15:4511–4525
Zarghani M, Akhlaghinia B (2016) Bull Chem Soc Jpn. doi:10.1246/bcsj.20160163
Jafarpour M, Rezapour E, Ghahramaninezhad M, Rezaeifard A (2014) New J Chem 38:676–682
Lomoschitz ChJ, Feichtenschlager B, Moszner N, Puchberger M, Muller K, Abele M, Kickelbickn G (2011) Langmuir 27:3534–3540
Du Q, Zhang W, Ma H, Zheng J, Zhou B, Li Y (2012) Tetrahedron 68:3577–3584
Jayakumar S, Ananthapadmanabhan PV, Thiyagarajan TK, Perumal K, Mishra SC, Suresh G, Su LT, Tok AIY (2013) Mater Chem Phys 140:176–182
Kazemi F, Saberi A, Malek-Ahmadi S, Sohrabi S, Rezaie HR, Taheriri M (2011) Ceramics-Silikáty 55:26–30
Veisi H, Gholami J, Ueda H, Mohammadi P, Noroozi M (2015) J Mol Catal A Chem 396:216–223
Farjadian F, Hosseini M, Ghasemi S, Tamami B (2015) RSC Adv 5:79976–79987
Ozawa F, Kubo A, Hayashi T (1992) Chem Lett 11:2177–2180
Iranpoor N, Firouzabadi H, Azadi R (2007) Eur J Org Chem 2007:2197–2201
Ebrahimzadeh F, Tamami B (2015) Phosphorus Sulfur Silicon Relat Elem 190:144–157
Iranpoor N, Firouzabadi H, Motevalli S, Talebi M (2012) J Organomet Chem 708:118–124
Ukisu Y (2015) Reac Kinet Mech Cat 114:385–394
Shen C, Wang YJ, Xu JH, Wang K, Luo GS (2012) Langmuir 28:7519–7527
Adlim M, Abu Bakar M, Liew KY, Ismail J (2004) J Mol Catal A Chem 212:141–149
Wang X, Lu G, Guo Y, Qiao D, Zhang Z, Guo Y, Li C (2008) Chin J Catal 29:1043–1050
Razavi N, Akhlaghinia B (2015) RSC Adv 5:12372–12381
Ghodsinia SSE, Akhlaghinia B (2015) RSC Adv 5:49849–49860
Zarei Z, Akhlaghinia B (2015) Chem Pap 69:1421–1437
Zarghani M, Akhlaghinia B (2015) Appl Organometal Chem 29:683–689
Zarghani M, Akhlaghinia B (2016) RSC Adv 6:31850–31860
Razavi N, Akhlaghinia B (2016) New J Chem 40:447–457
Jahanshahi R, Akhlaghinia B (2016) RSC Adv 6:29210–29219
Ghodsinia SSE, Akhlaghinia B (2016) RSC Adv 6:63613–63623
Yu K, Sommer W, Richardson JM, Weck M, Jones CW (2005) Adv Synth Catal 347:161–171
Khalafi-Nezhad A, Panahi F (2013) J organomet chem 741:7–14
Panahi F, Zarnaghash N, Khalafi–Nezhad A (2016) New J Chem 40:1250–1255
Acknowledgements
The authors gratefully acknowledge the partial support of this study by Ferdowsi University of Mashhad Research Council (Grant No. p/3/32111).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Razavi, N., Akhlaghinia, B. & Jahanshahi, R. Aminophosphine Palladium(0) Complex Supported on ZrO2 Nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media. Catal Lett 147, 360–373 (2017). https://doi.org/10.1007/s10562-016-1944-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1944-x




