Abstract
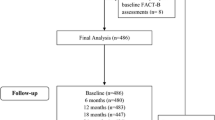
Health-related quality of life (HRQOL), symptoms of depression, and adverse events (AEs) were compared between Japanese postmenopausal patients with hormone-sensitive breast cancer (BC) who received adjuvant tamoxifen, exemestane, or anastrozole in an open-labeled, randomized, multicenter trial designated as the National Surgical Adjuvant Study of Breast Cancer (N-SAS BC) 04 substudy of the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial. During the first year of treatment, HRQOL and symptoms of depression were analyzed using the Functional Assessment of Cancer Therapy-Breast (FACT-B) and its Endocrine Symptom Subscale (ES), and the Center for Epidemiologic Studies Depression Scale (CES-D), respectively. In addition, predefined AEs were analyzed. A total of 166 eligible patients were randomly assigned to receive adjuvant tamoxifen, exemestane, or anastrozole. FACT-B scores increased after treatment began and remained significantly higher in the tamoxifen group than in the exemestane group or anastrozole group during the first year (P = 0.045). FACT-B scores were similar in the exemestane group and anastrozole group. ES scores and CES-D scores were similar in all treatment groups. Arthralgia and fatigue were less frequent, but vaginal discharge was more frequent in the tamoxifen group than in the exemestane group or anastrozole group. HRQOL was better in Japanese postmenopausal women treated with tamoxifen than those treated with exemestane or anastrozole. HRQOL and AEs were similar with exemestane and anastrozole. Given the results of the TEAM trial, upfront use of tamoxifen followed by an aromatase inhibitor (AI) may be an important option for adjuvant endocrine therapy in Japanese postmenopausal women.


Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- AI:
-
Aromatase inhibitor
- ATAC:
-
Arimidex, tamoxifen, alone or in combination
- BC:
-
Breast cancer
- BCS:
-
Breast cancer subscale
- BMD:
-
Bone mineral density
- CES-D:
-
Center for epidemiologic studies depression scale
- CYP:
-
Cytochrome P450
- DFS:
-
Disease-free survival
- ES:
-
Endocrine symptom subscale
- FACT-B:
-
Functional assessment of cancer therapy-breast
- FACT-G:
-
Functional assessment of cancer therapy-general
- HRQOL:
-
Health-related quality of life
- N-SAS BC:
-
National Surgical Adjuvant Study of Breast Cancer
- TEAM:
-
Tamoxifen exemestane adjuvant multinational
References
Parkin DM, Bray FI, Devesa SS (2001) Cancer burden in the year 2000. The international picture. Eur J Cancer 37(Suppl 8):S4–S66
Shibuta K, Ueo H, Furusawa H et al (2011) The relevance of intrinsic subtype to clinicopathological features and prognosis in 4,266 Japanese women with breast cancer. Breast Cancer 18:292–298
Goss PE (2003) Emerging role of aromatase inhibitors in the adjuvant setting. Am J Clin Oncol 26:S27–S33
Lin NU, Winer EP (2008) Advances in adjuvant endocrine therapy for postmenopausal women. J Clin Oncol 26:798–805
Kaufmann M, Jonat W, Hilfrich J et al (2007) Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 study. J Clin Oncol 25:2664–2670
Coombes RC, Kilburn LS, Snowdon CF et al (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369:559–570
Coates AS, Keshaviah A, Thurlimann B et al (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25:486–492
Forbes JF, Cuzick J, Buzdar A et al (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9:45–53
Ziller V, Kalder M, Albert US et al (2009) Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol 20:431–436
Fallowfield L, Cella D, Cuzick J et al (2004) Quality of life of postmenopausal women in the arimidex, tamoxifen, alone or in combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 22:4261–4271
Whelan TJ, Goss PE, Ingle JN et al (2005) Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol 23:6931–6940
Fallowfield LJ, Bliss JM, Porter LS et al (2006) Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol 24:910–917
Cella D, Fallowfield L, Barker P et al (2006) Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat 100:273–284
Mamounas EP, Jeong JH, Wickerham DL et al (2008) Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 26:1965–1971
Hadji P (2008) Menopausal symptoms and adjuvant therapy-associated adverse events. Endocr Relat Cancer 15:73–90
Jones SE, Cantrell J, Vukelja S et al (2007) Comparison of menopausal symptoms during the first year of adjuvant therapy with either exemestane or tamoxifen in early breast cancer: report of a Tamoxifen Exemestane Adjuvant Multicenter trial substudy. J Clin Oncol 25:4765–4771
Goss PE, Ingle JN, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802
Buzdar A, Howell A, Cuzick J et al (2006) Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 7:633–643
Miller WR, Bartlett J, Brodie AM et al (2008) Aromatase inhibitors: are there differences between steroidal and nonsteroidal aromatase inhibitors and do they matter? Oncologist 13:829–837
van de Velde CJ, Rea D, Seynaeve C et al (2011) Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 377:321–331
Hozumi Y, Suemasu K, Takei H et al (2011) The effect of exemestane, anastrozole, and tamoxifen on lipid profiles in Japanese postmenopausal early breast cancer patients: final results of National Surgical Adjuvant Study BC 04, the TEAM Japan sub-study. Ann Oncol 22:1777–1782
Aihara T, Suemasu K, Takei H et al (2011) Effects of exemestane, anastrozole and tamoxifen on bone mineral density and bone turnover markers in postmenopausal early breast cancer patients: results of N-SAS BC 04, the TEAM Japan substudy. Oncology 79:376–381
Brady MJ, Cella DF, Mo F et al (1997) Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 15:974–986
Fallowfield LJ, Leaity SK, Howell A et al (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55:189–199
Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401
Shimozuma K, Morita S, Ohsumi S et al (2004) Predictors of health-related quality of life of breast cancer patients after surgery in Japan (Women’s Health Outcome Study [WHOS]-01). Qual Life Res 13:1518
Fumimoto H, Kobayashi K, Chang CH et al (2001) Cross-cultural validation of an international questionnaire, the general measure of the functional assessment of cancer therapy scale (FACT-G), for Japanese. Qual Life Res 10:701–709
Shima S, Shikano T, Kitamura T et al (1985) New self-rating scales for depression [in Japanese]. Seishin Igaku 27:717–723
Cella DF, Tulsky DS, Gray G et al (1993) The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Japan Clinical Oncology Group (2001) National Cancer Institute—Common Toxicity Criteria (NCI-CTC Version 2.0, April 30, 1999), 2001/9/17 update. http://www.jcog.jp/doctor/tool//ctcindex.html. Accessed 23 May 2011
Ohsumi S, Shimozuma K, Ohashi Y et al (2011) Health-related quality of life and psychological distress of breast cancer patients after surgery during a phase III randomized trial comparing continuation of tamoxifen with switching to anastrozole after adjuvant tamoxifen for 1–4 years: N-SAS BC 03. Breast Cancer Res Treat 127:143–152
Day R, Ganz PA, Costantino JP et al (1999) Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Clin Oncol 17:2659–2669
Lee KC, Ray GT, Hunkeler EM et al (2007) Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics 48:205–210
Schilder CM, Eggens PC, Seynaeve C et al (2009) Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol 48:76–85
Baum M, Buzdar A, Cuzick J et al (2003) ATAC (arimidex, tamoxifen alone or in combination) trialists’ group: anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (arimidex, tamoxifen alone or in combination) trial efficacy and safety update analyses. Cancer 98:1802–1810
Moy B, Tu D, Pater JL et al (2006) Clinical outcomes of ethnic minority women in MA.17: a trial of letrozole after 5 years of tamoxifen in postmenopausal women with early stage breast cancer. Ann Oncol 17:1637–1643
Avis NE, Kaufert PA, Lock M et al (1993) The evolution of menopausal symptoms. Baillieres Clin Endocrinol Metab 7:17–32
Avis NE, Stellato R, Crawford S et al (2001) Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 52:345–356
Tchen N, Juffs HG, Downie FP et al (2003) Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol 21:4175–4183
Stearns V, Johnson MD, Rae JM et al (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Goetz MP, Rae JM, Suman VJ et al (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23:9312–9318
Ingelman-Sundberg M (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 5:6–13
Goetz MP, Kamal A, Ames MM (2008) Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther 83:160–166
Acknowledgments
We thank all the patients, investigators, and clinical research coordinators who participated in N-SAS BC 04. Data management and statistical analysis services were provided by the non-profit organization Japan Clinical Research Support Unit (Tokyo, Japan), and medical writing services were provided by Statcom Co., Ltd. (Tokyo, Japan).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was funded by the Comprehensive Support Project for Oncology Research (CSPOR) and for Health Outcomes Research (CSP-HOR) of the Public Health Research Foundation. The corporate and individual sponsors of this study are listed on the CSPOR website (http://www.csp.or.jp/cspor/kyousan_e.html). The pharmaceutical manufacturer/distributor who had provided financial contributions as a corporate sponsor took no part in this study, other than providing information relevant to proper use of the study drugs. All decisions concerning the planning, implementation, and publication of this study were made by the Executive Committee.
Rights and permissions
About this article
Cite this article
Takei, H., Ohsumi, S., Shimozuma, K. et al. Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04). Breast Cancer Res Treat 133, 227–236 (2012). https://doi.org/10.1007/s10549-011-1943-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1943-y