Abstract
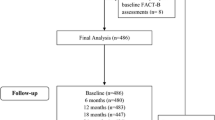
The impact of treatment on health-related quality of life (HRQoL) is an important consideration in the adjuvant treatment of operable breast cancer. Here we report mature HRQoL outcomes from the ATAC trial, comparing anastrozole with tamoxifen as primary adjuvant therapy for postmenopausal women with localized breast cancer. Patients completed the Functional Assessment of Cancer Therapy-Breast (FACT-B) questionnaire plus endocrine subscale (ES) at baseline, 3 and 6 months, and every 6 months thereafter. Baseline characteristics in the HRQoL sub-protocol were well balanced between the anastrozole (n = 335) and tamoxifen (n = 347) groups in the primary analysis population. As with previously published results at 2 years, there was no statistically significant difference in the Trial Outcome Index of the FACT-B, the primary endpoint of the study, between treatments at 5 years. There were no statistically significant differences between treatment groups in ES total scores. Consistent with the 2-year analysis, there were differences between treatment groups in patient-reported side effects: diarrhea (anastrozole 3.1% vs. tamoxifen 1.3%), vaginal dryness (18.5% vs. 9.1%), diminished libido (34.0% vs. 26.1%), and dyspareunia (17.3% vs. 8.1%) were significantly more frequent with anastrozole compared to tamoxifen. Dizziness (3.1% vs. 5.4%) and vaginal discharge (1.2% vs. 5.2%) were significantly less frequent with anastrozole compared to tamoxifen. In this, the first report of HRQoL over 5 years of initial adjuvant therapy with an aromatase inhibitor, we conclude that anastrozole and tamoxifen had similar impacts on HRQoL, which was maintained or slightly improved during the treatment period for both groups.
Similar content being viewed by others
References
Early Breast Cancer Trialistsȁ9 Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
The ATAC Trialistsȁ9 Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
ATAC Trialistsȁ9 Group (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 yearsȁ9 adjuvant treatment for breast cancer. Lancet 365:60–62
Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, Liu S, Shepherd LE, Palmer M, Robert NJ, Martino S, Muss HB (2005) Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol 23:6931–6940
Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A (2004) Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol 22:4261–4271
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997) Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 15:974–986
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Research and Treatment 55:189–199
Eton DT, Cella D, Yost KJ, Yount SE, Peterman AH, Neuberg DS, Sledge GW, Wood WC (2004) A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol 57:898–910
Yost KJ, Eton DT (2005) Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof 28:172–191
Fallowfield L, Atkins L, Catt S, Cox A, Coxon C, Langridge C, Morris R, Price M (2006) Patientsȁ9 preference for administration of endocrine treatments by injection or tablets: results from a study of women with breast cancer. Ann Oncol 17:205–210
Demissie S, Silliman RA, Lash TL (2001) Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol 19:322–328
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Fallowfield LJ, Bliss JM, Porter LS, Price MH, Snowdon CF, Jones SE, Coombes RC, Hall E (2006) Quality of Life in the Intergroup Exemestane Study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol. 24:910–917
Cuzick J (2003) Aromatase inhibitors in prevention – data from the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial and the design of IBIS-II (the second International Breast Cancer Intervention Study). Recent Results Cancer Res 163:96–103
National Comprehensive Cancer Network: NCCN practice guidelines in oncology v.1.2005: breast cancer. http://www.nccn.org 2005
Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Bryant J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR (2005) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol 23:619–629
Acknowledgements
We thank the patients for their participation in the trial and all those listed below. Members of the Writing Group for this paper are asterisked. We thank Dr JH Bull, from Complete Medical Communications, who provided medical writing support on behalf of AstraZeneca.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix
Appendix
The ATAC Trialists group
ATAC Steering Committee
Prof J Adams: University of Manchester, Manchester, UK; Professor M Baum: University College London, London, UK; Prof AR Bianco: Universita Degli Studi Di Napoli Federico II, Napoli Italy; Prof A Buzdar (Chairman): The University of Texas, M.D. Anderson Cancer Center, Houston, USA; *Prof D Cella: Northwestern University, Evanston, Illinois, USA; Dr M Coibion: Institut Bordet, Bruxelles, Belgium; Professor R Coleman: Cancer Research Centre, Weston Park Hospital, Sheffield, UK; Dr M Constenla: Hospital Montecelo, Pontevedra, Spain; *Prof J Cuzick: Cancer Research UK, London, UK; Prof Dr W Distler: Universitätsklinikum Dresden, Dresden, Germany; Prof M Dowsett: The Royal Marsden Hospital, London, UK; Mr S Duffy: St Jamesȁ9s University Hospital, Leeds, UK; Prof R Eastell: University of Sheffield, Sheffield, UK; *Prof LJ Fallowfield, University of Sussex, Brighton, UK; Prof J Forbes: Newcastle Mater Misericordiae Hospital, NSW, Australia; Prof WD George: Beatson Oncology Centre, Western Infirmary, Glasgow, UK; Sr J Grey: Belfast City Hospital, Belfast, UK; Dr J-P Guastalla: Centre Léon Bérard, Lyon, France; Mr R Hellmund, Dr G Hoctin-Boes: AstraZeneca Pharmaceuticals, Wilmington, USA; Mrs J Houghton, Dr N Williams: Clinical Trials Group of the Department of Surgery, UCL, London, UK; *Prof A Howell: Christie Hospital, Manchester, UK; Prof Dr JGM Klijn: Dr Daniel den Hoed Kliniek and University Hospital, Rotterdam, The Netherlands; *Dr GY Locker: Evanston Northwestern Healthcare, Northwestern University Feinberg School of Medicine, Evanston, IL, USA; Dr J Mackey: Cross Cancer Institute, Edmonton, Alberta, Canada; *Prof RE Mansel: University of Wales College of Medicine, Cardiff, UK; Prof JM Nabholtz: Hartman Oncology Institute, Levallois-Perret, France; Dr T Nagykalnai: Uzsoki U Hospital, Budapest, Hungary; Dr A Nicolucci: GIVIO Co-ordinating Centre, Consorzio Mario Negri Sud, Centro Di Ricerchi Farmacologichi, E Biomedichi, Chieta, Italy; Dr U Nylen: Radiumhemmet, Karolinska Sjukhuset, Stockholm, Sweden; Mr R Sainsbury: University College London (UCL), London, UK; Dr F Sapunar, Dr VJ Suarez-Mendez: AstraZeneca Pharmaceuticals, Macclesfield, UK; Prof JS Tobias: The Meyerstein Institute of Clinical Oncology, Middlesex Hospital, London, UK.
Principal and main co-investigators in the ATAC quality-of-life sub-protocol
Australia—P Grantley-Gill: Royal Adelaide Hospital, Adelaide, SA, Australia; J Beith: Royal Prince Alfred Hospital, Camperdown, NSW, Australia; S Della-Fiorentina, A Goldrick: Liverpool Hospital, Liverpool, NSW, Australia; G Richardson: Monash Medical Centre, East Bentleigh, VIC, Australia; G Toner: Peter MacCallum Cancer Institute, Melbourne, VIC, Australia; R Snyder: St Vincentȁ9s Hospital, Fitzroy, VIC, Australia; M Friedlander: Prince of Wales Hospital, Randwick, NSW, Australia; J Forbes: Newcastle Mater Misericordiae Hospital, Waratah, NSW, Australia. Canada—R Simard: Complexe Hospitalier de la Sagami, Chicoutimi, Quebec, Canada; JR MacKey (J-M Nabholtz former PI): Cross Cancer Institute, Edmonton, Alberta, Canada; WS Lofters: Kingston Regional Cancer Center, Kingston, Ontario, Canada; LC Panasci: McGill Department of Oncology, Montreal, Quebec, Canada; LA Zibdawi: Southlake Regional Health Centre, Newmarket, Ontario, Canada; RF Wierzbicki: Peterborough Regional Health Center Oncology Clinic, Peterborough, Ontario, Canada; J Robert: CHA Pavillion St Sacrement, Quebec City, Quebec, Canada; S Lebel (M Potvin former PI): Hospital Laval, Ste Foy, Quebec, Canada; M Jancerwicz: Allan Blair Cancer Center, Regina, Saskatchewan, Canada; A Sami (BA Walley former PI): Saskatoon Cancer Center, University of Saskatoon, Saskatoon, Saskatchewan, Canada; PK Ganguly: H Bliss Murphy Cancer Centre, St Johns, Newfoundland, Canada; Y Rahim: Toronto East General Hospital, Toronto, Ontario, Canada; JJ Wilson: Humber River Regional Hospital, Weston, Ontario, Canada. France—D Serin, Y Goubely-Brewer: Clinique Ste Catherine, Avignon, France; R Coquard (P Martin former PI), Dr Portois, Dr Guillot: Clinique St Jean, Lyon, France; M Rios: Centre Alexis Vautrin, Nancy, France; J-M Vannetzel: Centre Chirurgical Henri Hartmann, Neuilly sur Seine, France. Italy—F Recchia, S De Filippis: Ospedale Civile, Avezzano, Italy; A Martoni, E Piana: Ospedale S Orsola-M Malpighi, Bologna, Italy; M Giordano, G Luchena: Ospedale S Anna, Como, Italy; A Nuzzo, L Laudadio: Ospedale Floraspe Renzetti, Lanciano, Italy; G Gardani: Ospedale S Gerardo, Monza (Milan), Italy; A De Matteis: Istituto Nazionale Tumori “G Pascale”, Napoli, Italy; AR Bianco: Università Federico II, Napoli, Italy; G Brignone, L Mesi: Ospedale Oncologico M Ascoli, Palermo, Italy; M Mattarei: Ospedale Civile Piazzale, Rovereto, Italy; A Farris: Istituto Clinica Medica, Sassari, Italy. New Zealand—I Campbell: Breast Care Centre, Waikato Hospital, Hamilton, New Zealand. South Africa—L Goedhals, L Smith: Nationale Hospital, Bloemfontein, South Africa; I Werner, E Murray: Groote Schuur Hospital, Cape Town, South Africa; D Hacking: Durban Oncology Centre, Westridge, Durban, South Africa; D Vorobiof, M Chasen: Sandton Oncology Centre, Sandton, Johannesburg, South Africa. Spain—A Barnadas Molins: Hosp. Germans Trias i Pujol, Badalona (Barcelona), Spain; L Calvo: Hosp. Juan Canalejo, La Coruña, Spain; JR Mel, G Quintero: Hosp. Xeral Calde de Lugo, Lugo, Spain; P España, R Cubedo: Cervera: Hosp. Puerta de Hierro, Madrid, Spain; P Aramburo: Hosp. Ruber Internacional, Madrid, Spain; JJ Valerdi: Hosp. de Navarra, Pamplona, Spain; J Lizon y Giner: Hosp. San Juan, San Juan (Alicante), Spain; MD Menendez: Hosp. Provincial de Conxo, Santiago de Compostela, Spain; G Huidobro Vence: Hosp. Meixoeiro, Vigo, Spain. The Netherlands—R Boom: Ziekenhuis Amstelveen, Amstelveen, The Netherlands; MB Polēe, H de Graaf: Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands; F Erdkamp: Maasland Ziekenhuis, Sittard, The Netherlands; A van Reisen: Ziekenhuis Zeeuws Vlaanderen, loc. de Honte, Terneuzen, The Netherlands; C van der Heul: St Elisabeth Ziekenhuis, Tilburg, The Netherlands; L Kerhofs (G-J Creemers former PI): Ziekenhuis Walcheren, Vlissingen, The Netherlands; J Coenen: Isala Klinieken, loc. Sophia, Zwolle, The Netherlands. UK—A Hutcheon, T Sarkar: Aberdeen Royal Infirmary, Aberdeen, UK; T Bates, N Griffiths: South Kent Hospitals NHS Trust, William Harvey Hospital, Ashford, UK; N Stuart, D Crawford: Gwynedd Hospital NHS Trust, Bangor, UK; B Lavery, E Sugden: Horton General Hospital, Banbury, UK; S Dolan, A Wilkinson (G Odling-Smee former PI): Belfast City Hospital NHS Trust, Belfast, UK; T Hickish, A Skene: Royal Bournemouth & Christchurch NHS Trust, Royal Bournemouth Hospital, Bournemouth, UK; *L Fallowfield, Cancer Research UK Psychosocial Oncology Group, Brighton & Sussex Medical School, University of Sussex, Brighton, UK; R Mansel, H Sweetland: University Hospital of Wales NHS Trust, Cardiff, UK; D Cameron: Western General Hospital, Edinburgh, UK; WD George, G Fraser: Western Infirmary, Glasgow, UK; B Lavery, A Harris, D Talbot, A Jones: Oxford Radcliffe Hospital NHS Trust, John Radcliffe Hospital, Headington, UK; B Lavery: South Bucks NHS Trust, Wycombe General Hospital, High Wycombe, UK; J LeVay, T Archer: Ipswich Hospitals NHS Trust, Ipswich, UK; A Nejim, I Hutchinson: Airedale General Hospital, Keighley, UK; K Horgan, D Dodwell: Leeds General Infirmary, Leeds, UK; C Holcombe: Royal Liverpool University Hospital NHS Trust, Royal Liverpool Hospital, Liverpool, UK; J Tobias, M Gaze (M Baum former PI): UCL Hospitals NHS Trust, The Middlesex Hospital, London, UK; J Mansi, N Sacks: St Georgesȁ9 NHS Trust Hospital, Tooting, London, UK; A Jones, T Davidson: Royal Free Hampstead NHS Trust, The Royal Free Hospital, London, UK; A Wardley (A Howell former PI): Withington Hospital, Manchester, UK; C Griffith, A Griffiths: Newcastle Hospitals NHS Trust, Royal Victoria Infirmary, Newcastle-upon-Tyne, UK; C Gaffney: Glan Hafren NHS Trust, Royal Gwent Hospital, Newport, UK; JR Robertson: Nottingham City Hospital NHS Trust, Nottingham, UK; D Pinto: Sperrin Lakelands Health & Social Care Trust, Tyrone County Hospital, Omagh, UK; B Lavery, A Harris, D Talbot, A Jones: Oxford Radcliffe Hospital NHS Trust, The Churchill Hospital, Oxford, UK; R Coleman, S Kohlhardt: Weston Park Hospital, Sheffield, UK; B Harrison: Northern General Hospital, Sheffield, UK; R Agrawal: Royal Shrewsbury NHS Trust, Shrewsbury, UK; C Hennessy (A Peel former PI): North Tees General Hospital, Stockton-on-Tees, UK; A Paterson (S Kelly former PI), K Stepp: Royal Cornwall Hospitals NHS Trust, Treliske Hospital, Truro, UK; R Grieve, T Waterworth: Walsgrave Hospitals NHS Trust, Walsgrave, UK; P Barrett-Lee: Velindre NHS Trust Hospital, Whitchurch, UK; D Berstock, R Errington: Wirral Hospital NHS Trust, Wirral, UK; A Salman, A Johri: Worthing Southlands Hospital NHS Trust, Worthing Hospital, Worthing, UK; S Goodman (EC Whipp former PI), G Sparrow: East Somerset NHS Trust, Yeovil District Hospital, Yeovil, UK; S Nicholson: York District Hospital, York, UK. USA—RO Kerr: Southwest Regional Cancer Center, Austin, TX, USA; GP Miletello: Hematology/Oncology Clinic, Baton Rouge, LA, USA; LR Laufman: Columbus CCOP, Columbus, OH, USA; GY Locker: Evanston Northwestern Health Care, Kellogg Cancer Care Center, Evanston, IL, USA; RH Clark: Hematology/Oncology Associates, Jackson, MI, USA; K B Pendergrass: Kansas City Internal Medicine, Kansas City, MO, USA; JJ Sternberg: Arkansas Oncology Associates, 1000 N. University, Little Rock, AR, USA; W Bate: North Iowa Mercy Health Center, Mason City, IA, USA; PV Pickens: Abington Hematology–Oncology, Meadowbrook, PA, USA; MW Meshad: Providence Hospital, Mobile, AL, USA; KK Boatman: INTERGIS Oncology Services, Oklahoma City, OK, USA; MS Roberts: Hematology & Oncology Associates Ltd, Phoenix, AZ, USA; JI Spector: Berkshire Hematology Oncology, Pittsfield, MA, USA; MA Deutsch: Raleigh Hematology Oncology, Raleigh, NC, USA; P Bushunow (M Brower former PI): Rochester General Hospital, Rochester, NY, USA; WR Edwards: ACT Medical Group, 2473 MacFarland Road, Rockford Clinic, Rockford, IL, USA; IA Jaiyesimi: Cancer Care Associates PC, Royal Oak, MI, USA; FC Kass: Santa Barbara Hematology–Oncology Medical Group, Santa Barbara, CA, USA; PD Byeff: Hematology/Oncology, Southington, CT, USA; *D Cella, Center on Outcomes Research and Education (CORE), Evanston Northwestern Healthcare and Northwestern University, IL, USA; KL Hoelzer: Springfield Clinic, Springfield, IL, USA; EP Lester: Oncology Care Associates PLLC, St Joseph, MI, USA; AP Lyss: Missouri Baptist Cancer Center, St Louis, MO, USA; EP Gelman (DF Hayes former PI) (MJC Ellis former PI): Georgetown University Medical Lombardi Cancer Center, Washington, DC, USA; MA Vukelich: Kettle Moraine Oncology SC, West Bend, WI, USA; K Seetharaman (LL Stolbach former PI): St Vincentȁ9s Hospital, Worcester, MA, USA.
International Project Team
A Doe, Dr F Sapunar, Dr VJ Suarez-Mendez: AstraZeneca Pharmaceuticals, Macclesfield, UK; E Foster: ISD Cancer Clinical Trials Team, Edinburgh, UK; J Houghton, N Williams: Clinical Trials Group of the Department of Surgery, UCL, London, UK; A Nicolucci: Mario Negri Institute, Chieta, Italy; S Pollard: Clinical Trials Research Unit, Leeds, UK.
Data Monitoring Committee
Dr M Buyse: International Institute for Drug Development (ID squared), Brussels, Belgium; *Prof J Cuzick (Independent statistician), Cancer Research UK, London, UK; Dr R Margolese: McGill University, The Sir Mortimer B Davis Jewish General Hospital, Montreal, Québec, Canada; Prof JJ Body: Institute J Bordet, Bruxelles, Belgium.
Collaborative/Operational Groups
JF Forbes (Group Co-ordinator), JK Wakeham (Study Coordinator): Australian New Zealand Breast Cancer Trials Group Operations Office; S de Placido (Study Co-ordinator), C Carlomagno (Study Co-ordinator): Universita Degli Studi Di Napoli Federico II, Italy; A Nicolucci (Group Co-ordinator), M Belfiglio (Study Co-ordinator), M Valentini (Study Co-ordinator): GIVIO Group, Consorzio Mario Negri Sud, Italy; E Foster (Principal Trial Co-ordinator and CCTT contact): ISD Cancer Clinical Trials Team, Edinburgh, UK, C Lacey (Trial monitor): North West Breast Group, Burnley, Lancashire, UK; S Pollard (Head of Pharmaceutical Collaboration): Clinical Trials Research Unit, University of Leeds, Leeds, UK; J Houghton (Senior Lecturer in Clinical Trials and CTG contact), N Williams (Trial Co-ordinator): Clinical Trials Group of the Department of Surgery, UCL, London, UK.
Rights and permissions
About this article
Cite this article
Cella, D., Fallowfield, L., Barker, P. et al. Quality of Life of Postmenopausal Women in the ATAC (“Arimidex”, Tamoxifen, Alone or in Combination) Trial after Completion of 5 years' Adjuvant Treatment for Early Breast Cancer. Breast Cancer Res Treat 100, 273–284 (2006). https://doi.org/10.1007/s10549-006-9260-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9260-6