Abstract
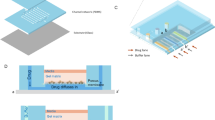
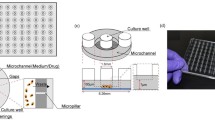
A high-throughput, microfluidic flow cell array (MFCA) system has been modified to enable drug screening against small-volume cell-, and tissue cultures. The MFCA is composed of a 3D channel network that simultaneously flows fluids through forty-eight 830 μm by 500 μm flow cells, which physically divide and fluidically seal an existing culture into multiple compartments when docked onto the surface of a cell or tissue culture dish. The modified system provides temperature (37 °C) and CO2/pH level controls, while continuously flowing solutions (media or other liquid such as drug suspensions) over the cells/tissues. These assays were enhanced and validated using inverted microscopy and fluorescent staining techniques which also allow real time viability and toxicity assessments. This work presents the results of this new generation in vitro drug testing assay performed using this modified MFCA system. This setup allows the testing of 48 drug combinations on 48 different cell-, tissue specimen at once under flow conditions. All 48 flow cells were utilized to test 5 different concentrations of cisplatin (CDDP). CDDP solutions in various concentrations were continually flowed over cultured human ovarian cancer cells for 48 h. Viability assessments were performed using red-orange calcein and SYTOX ® Green nucleic acid stains. Cells were imaged at the beginning and end of the experiment (48 h). In order to compare and validate MFCAs suitability as drug screening assay, MTT assays were performed on cells. We found that both, MTT and MFCA assays generated dose-response curves with similar profiles. Innovative advantages of the MFCA system include the ability of handling smaller amounts of solutions compared to conventional and current state of the art drug screening and cell viability/toxicity methods. It also provides the ability to continually deliver fresh solution to the cell samples, while eliminating wastes that are produced. Based on our here reported findings MFCA may have a strong potential of providing a more physiological model than current state of the art static MTT assays.






Similar content being viewed by others
References
M. Astolfi et al., Micro-dissected tumor tissues on chip: an ex vivo method for drug testing and personalized therapy. Lab Chip 16, 312–325 (2016)
L. Avraham-chakim et al., Fluid-flow induced wall shear stress and epithelial ovarian cancer peritoneal spreading. PLoS One 8, 1–8 (2013)
M. Bookman, A. First-line, Chemotherapy in epithelial ovarian cancer. Clin. Obstet. Gynecol. 55, 96–113 (2012)
C.P. Carmignani, T.A. Sugarbaker, C.M. Bromley, P.H. Sugarbaker, Intraperitoneal cancer dissemination: Mechanisms of the patterns of spread. Cancer Metastasis Rev. 22, 465–472 (2003)
D.A. Chang-Yen, D.G. Myszka, B.K. Gale, A novel PDMS microfluidic spotter for fabrication of protein chips and microarrays. J. Microelectromech. Syst. 15, 1145–1151 (2006)
S.N. Davidoff et al., The submerged printing of cells onto a modified surface using a continuous flow Microspotter. J. Vis. Exp. 86, 1–6 (2014)
M.A. Eddings et al., ‘spot and hop’: Internal referencing for surface plasmon resonance imaging using a three-dimensional microfluidic flow cell array. Anal. Biochem. 385, 309–313 (2009)
L. Florento et al., Comparison of cytotoxic activity of anticancer drugs against various human tumor cell lines using In Vitro cell-based approach. Int. J. Biomed. Sci. 8, 76–80 (2012)
M. Harper, The Cost of Creating A New Drug Now $5 Billion, Pushing Big Pharma To Change. (Forbes, 2013), http://www.forbes.com/sites/matthewherper/2013/08/11/how-the-staggering-cost-of-inventing-new-drugs-isshaping-the-future-of-medicine/. Accessed 10 July 2015
P.J. Hung, P.J. Lee, P. Sabounchi, R. Lin, L.P. Lee, Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol. Bioeng. 89, 1–8 (2005a)
P.J. Hung et al., A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array. Lab Chip 5, 44–48 (2005b)
M. Johnson, G. Liddiard, M. Eddings, B. Gale, Bubble inclusion and removal using PDMS membrane-based gas permeation for applications in pumping, valving and mixing in microfluidic devices. J. Micromech. Microeng. 95011, 9 (2009)
J. Kim et al., A programmable microfluidic cell array for combinatorial drug screening. Lab Chip 12, 1813 (2012)
M. Kim, R.J. Gillies, K.A. Rejniak, Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues. Pharmacol. Anti-Cancer Drugs 3, 278 (2013)
S. Natarajan et al., Continuous-flow microfluidic printing of proteins for array-based applications including surface plasmon resonance imaging. Anal. Biochem. 373, 141–146 (2008a)
S. Natarajan, A. Hatch, D. Myszka, B. Gale, Optimal conditions for protein array deposition using continuous flow. Anal. Chem. 80, 8561–8567 (2008b)
G. Omura et al., A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A gynecologic oncology group study. Cancer 57, 1725–1730 (1986)
S.C. Oppegard, K. Nam, J.R. Carr, S.C. Skaalure, D.T. Eddington, Modulating temporal and spatial oxygenation over adherent cellular cultures. PLoS One 4, e6891 (2009)
T.G. Papaioannou, C. Stefanadis, Vascular wall shear stress: basic principles and methods. Hell. J. Cardiol. 46, 9–15 (2005)
M. Rothbauer, D. Wartmann, V. Charwat, P. Ertl, Recent advances and future applications of microfluidic live-cell microarrays lab-on-a-chip organ-on-a-chip body-on-a-chip. Biotechnol. Adv. 33, 948–961 (2015)
S. Sato, H. Itamochi, Neoadjuvant chemotherapy in advanced ovarian cancer: Latest results and place in therapy. Ther. Adv. Med. Oncol. 6, 293–304 (2014)
M. Sittinger, O. Schultz, G. Keyszer, W.W. Minuth, G. R. Burmester, Artificial tissues in perfusion culture.pdf. Int. J. Artif. Organs. 20, 57–62 (1997)
American Cancer Society. Cancer Facts & Figures. (2015) Atlanta: AmericanCancer Society; 2015
H. Song, T. Chen, B. Zhang, Y. Ma, Z. Wang, An integrated microfluidic cell array for apoptosis and proliferation analysis induction of breast cancer cells. Biomicrofluidics 4, 44104 (2010)
S. A. Sundberg, High-throughput and ultra-high-throughput screening : solution- and cell-based approaches. Curr. Opin. Biotechnol. 11, 47–53 (2000)
M.H. Wu, J.P.G. Urban, Z. Cui, Z.F. Cui, Development of PDMS microbioreactor with well-defined and homogenous culture environment for chondrocyte 3-D culture. Biomed. Microdevices 8, 331–340 (2006)
M.-H. Wu, S.-B. Huang, G.-B. Lee, Microfluidic cell culture systems for drug research. Lab Chip 10, 939 (2010)
S.-T. Yang, J. Wang, R. Zang, I.-C. Tang, D. Li, Cell-based assays in high-throughput screening for drug discovery. Int. J. Biotechnol. Wellness Ind. (2012). doi:10.6000/1927-3037.2012.01.01.02
Acknowledgements
The authors gratefully acknowledge that this project was supported by NIH grant R43CA177146-01. Bruce Gale declares a financial interest in Wasatch Microfluidics, which has a license to the technology presented in this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(TXT 680 bytes)
Rights and permissions
About this article
Cite this article
Arellano, J., Howell, T., Gammon, J. et al. Use of a highly parallel microfluidic flow cell array to determine therapeutic drug dose response curves. Biomed Microdevices 19, 25 (2017). https://doi.org/10.1007/s10544-017-0166-3
Published:
DOI: https://doi.org/10.1007/s10544-017-0166-3