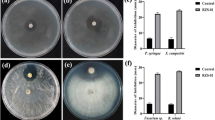
Abstract
Plant rhizobacteria have been successfully used as biocontrol agents against fungal phytopathogens. However, their potential to control two important avocado diseases, namely Fusarium dieback (FD) and Phytophthora root rot (PRR), has been poorly studied. FD is an emerging disease triggered by fungi associated with two ambrosia beetle species (Euwallacea fornicatus species complex), while PRR is caused by Phytophthora cinnamomi, a soil-borne oomycete. In the present work, the antifungal activity of bacteria isolated from avocado rhizosphere was tested in dual culture assays against Fusarium euwallaceae, Graphium euwallaceae and Graphium sp., causal agents of FD, and against P. cinnamomi. In 2015, rhizosphere soil samples of FD infested and non-infested avocado trees were collected from a commercial avocado orchard in Escondido, California. In an initial screening, 72 of the 168 assessed bacterial isolates reduced mycelial growth of F. euwallaceae by up to 46%. Eight bacterial isolates showing inhibition percentages larger than 40% were then selected for further antagonism assays against the other fungal pathogens. Five bacterial isolates, determined by 16S rDNA sequencing to belong to the Bacillus subtilis/Bacillus amyloliquefaciens species complex, successfully inhibited the mycelial growth of both Graphium species by up to 30%. The same isolates and an additional isolate identified as Bacillus mycoides, inhibited the growth of P. cinnamomi by up to 25%. This is the first report of avocado rhizobacteria with antifungal activity against pathogens responsible for FD and PRR in avocado.






Similar content being viewed by others
References
Abdallah RAB, Mokni-Tlili S, Nefzi A, Jabnoun-Khiareddine H, Daami-Remadi M (2016) Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Nicotiana glauca organs. Biol Control 97:80–88. https://doi.org/10.1016/j.biocontrol.2016.03.005
Ag Marketing Resource Center (AGMRC) (2014) http://www.agmrc.org/
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Bollet C, Gevaudan MJ, De Lamballerie X, Zandotti C, De Micco P (1991) A simple method for the isolation of chromosomal DNA from gram positive or acid-fast bacteria. Nucleic Acids Res 19:1955
Cawoy H, Debois D, Franzil L, De Pauw E, Thonart P, Ongena M (2015) Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotechnol 8:281–295. https://doi.org/10.1111/1751-7915.12238
Cazorla FM, Romero D, Pérez-García A, Lugtenberg BJJ, Vicente AD, Bloemberg G (2007) Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J Appl Microbiol 103:1950–1959. https://doi.org/10.1111/j.1365-2672.2007.03433.x
Chaves-López C, Serio A, Gianotti A, Sacchetti G, Ndagijimana M, Ciccarone C, Stellarini A, Corsetti A, Paparella A (2015) Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J Appl Microbiol 119:487–499. https://doi.org/10.1111/jam.12847
Coffey MD (1987) Phytophthora root rot of avocado: an integrated approach to control in California. Plant Dis 71:1046–1053
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:141–145. https://doi.org/10.1093/nar/gkn879
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678. https://doi.org/10.1016/j.soilbio.2009.11.024
Dunlap CA, Lueschow S, Carrillo D, Rooney AP (2017) Screening of bacteria for antagonistic activity against phytopathogens of avocados. Plant Gene 11:17–22. https://doi.org/10.1016/j.plgene.2016.11.004
Egamberdieva D, Wirth S, Behrendt U, Ahmad P, Berg G (2017) Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol 8:199. https://doi.org/10.3389/fmicb.2017.00199
Eskalen A, Gonzalez A, Wang DH, Twizeyimana M, Mayorquin JS, Lynch SC (2012) First report of a Fusarium sp. and its vector Tea Shot Hole Borer (Euwallacea fornicatus) causing Fusarium dieback on avocado in California. Plant Dis 96:1070. https://doi.org/10.1094/PDIS-03-12-0276-PDN
Eskalen A, Stouthamer R, Lynch SC, Rugman-Jones PF, Twizeyimana M, Gonzalez A, Thibault T (2013) Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Dis 97:938–951. https://doi.org/10.1094/PDIS-11-12-1026-RE
Fan B, Blom J, Klenk HP, Borriss R (2017) Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an “Operational Group B. amyloliquefaciens” within the B. subtilis species complex. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00022
Fiddaman PJ, Rossall S (1993) The production of antifungal volatiles by Bacillus subtilis. J Appl Bacteriol 74:119–126
Food and Agriculture Organization of the United Nations (FAOSTAT) (2015) http://www.fao.org/
García-Avila CDJ, Trujillo-Arriaga FJ, López-Buenfil JA, González-Gómez R, Carrillo D, Cruz LF, Ruiz-Galván I, Quezada-Salinas A, Acevedo-Reyes N (2016) First report of Euwallacea nr. fornicatus (Coleoptera: Curculionidae) in Mexico. Fla Entomol 99:555–556. https://doi.org/10.1653/024.099.0335
Hakizimana JD, Gryzenhout M, Coutinho TA, Van den Berg N (2011) Endophytic diversity in Persea americana (avocado) trees and their ability to display biocontrol activity against Phytophthora cinnamomi. In: Proceedings VII World Avocado Congress 2011, Cairns, Australia, 5–9 September 2011
Hall TA (1999) BioEdit: a friendly biological sequence alignment editor and analysis program for Window 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Holthorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Idris HA, Labuschagne N, Korsten L (2007) Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol Control 40:97–106. https://doi.org/10.1016/j.biocontrol.2006.07.017
Ji SH, Paul NC, Deng JX, Kim YS, Yun BS, Yu SH (2013) Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 41:234–242. https://doi.org/10.5941/MYCO.2013.41.4.234
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol msw054. https://doi.org/10.1093/molbev/msw054
Lynch SC, Twizeyimana M, Mayorquin JS, Wang DH, Na F, Kayim M, Kasson MT, Thu PQ, Bateman C, Rugman-Jones P, Hulcr J, Stouthamer R, Eskalen A (2016) Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov.—two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California. Mycologia 108:313–329. https://doi.org/10.3852/15-063
Mnif I, Hammami I, Triki MA, Azabou MC, Ellouze-Chaabouni S, Ghribi D (2015) Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus, Fusarium solani. Environ Sci Pollut Res 22:18137–18147. https://doi.org/10.1007/s11356-015-5005-6
Na F (2016) Identification of two novel fungal species associated with Kuroshio shot hole borer (Euwallacea sp.) and evaluation of novel biological control method to inhibit the fungal associates of the invasive ambrosia beetle species in California (Order No. 10153676). Available from ProQuest Dissertations and Theses Global. (1836080563). Retrieved from https://search.proquest.com/docview/1836080563?accountid=27949
Neupane S, Finlay RD, Alström S, Elfstrand M, Högberg N (2015) Transcriptional responses of the bacterial antagonist Serratia plymuthica to the fungal phytopathogen Rhizoctonia solani. Environ Microbiol Rep 7:123–127. https://doi.org/10.1111/1758-2229.12203
O’Donnell K, Sink S, Libeskind-Hadas R, Hulcr J, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE, Liyanage PNH, Eskalen A, Na F, Geiser DM, Bateman C, Freeman S, Mendel Z, Sharon M, Aoki T, Cossé AA, Rooney AP (2015) Discordant phylogenies suggest repeated host shifts in the Fusarium-Euwallacea ambrosia beetle mutualism. Fungal Genet Biol 82:277–290. https://doi.org/10.1016/j.fgb.2014.10.014
O’Donnell K, Libeskind-Hadas R, Hulcr J, Bateman C, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE, Liyanage PNH, Eskalen A, Lynch SC, Geiser DM, Freeman S, Mendel Z, Sharon M, Aoki T, Cossé AA, Rooney AP (2016) Invasive Asian Fusarium—Euwallacea ambrosia beetle mutualists pose a serious threat to forests, urban landscapes and the avocado industry. Phytoparasitica 44:435–442. https://doi.org/10.1007/s12600-016-0543-0
Pagliaccia D, Pond E, McKee B, Douhan GW (2013) Population genetic structure of Phytophthora cinnamomi associated with avocado in California and the discovery of a potentially recent introduction of a new clonal lineage. Phytopathology 103:91–97. https://doi.org/10.1094/PHYTO-01-12-0016-R
Prabhukarthikeyan SR, Manikandan R, Durgadevi D, Keerthana U, Harish S, Karthikeyan G, Raguchander T (2017) Bio-suppression of turmeric rhizome rot disease and understanding the molecular basis of tripartite interaction among Curcuma longa, Pythium aphanidermatum and Pseudomonas fluorescens. Biol Control 111:23–31. https://doi.org/10.1016/j.biocontrol.2017.05.003
Shaikh SS, Sayyed RZ (2015) Role of plant growth promoting rhizobacteria and their formulation in biocontrol of plant diseases. In: Arora NK (ed) Plant microbes symbiosis: applied facets. Springer, New Delhi
Stirling AM, Hayward AC, Pegg KG (1992) Evaluation of the biological control potential of bacteria isolated from a soil suppressive to Phytophthora cinnamomi. Australas Plant Path 21:133–142
Toerien J (2007) The Phytophthora challenge. Calif Avocado Soc Yearb 90:89–101
Tokpah DP, Li H, Wang L, Liu X, Mulbah QS, Liu H (2016) An assessment system for screening effective bacteria as biological control agents against Magnaporthe grisea on rice. Biol Control 103:21–29. https://doi.org/10.1016/j.biocontrol.2016.07.009
Umeda C, Eskalen A, Paine TD (2016) Polyphagous shot hole borer and Fusarium dieback in California. In: Paine T, Lieutier F (eds) Insects and diseases of Mediterranean forest systems. Springer International Publishing, New York
Vida C, Cazorla FM, de Vicente A (2017) Characterization of biocontrol bacterial strains isolated from a suppressiveness-induced soil after amendment with composted almond shells. Res Microbiol. https://doi.org/10.1016/j.resmic.2017.03.007
Wickham H (2011) The split-apply-combine strategy for data analysis. J Stat Softw 40:1–29. https://doi.org/10.18637/jss.v040.i01
Yang C, Crowley DE, Menge JA (2001) 16S rDNA fingerprinting of rhizosphere bacterial communities associated with healthy and Phytophtora infected avocado roots. FEMS Microbiol Ecol 35:129–136. https://doi.org/10.1111/j.1574-6941.2001.tb00796.x
Yin B, Scupham AJ, Menge JA, Borneman J (2004) Identifying microorganisms which fill a niche similar to that of the pathogen: a new investigative approach for discovering biological control organisms. Plant Soil 259:19–27
You MP, Sivasithamparam K, Kurtböke DI (1996) Actinomycetes in organic mulch used in avocado plantations and their ability to suppress Phytophthora cinnamomi. Biol Fertil Soils 22:237–242
Yuan J, Raza W, Shen Q, Huang Q (2012) Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl Environ Microbiol 78:5942–5944. https://doi.org/10.1128/AEM.01357-12
Acknowledgements
We thank Clemente García-Ávila and Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA) for facilitating the import of bacterial strains isolated from a California avocado orchard to Mexico. We also thank Eneas Aguirre, Diana Sánchez and Yonatan Escudero for their help with isolating and morphotyping bacterial isolates, and Ofelia Ferrera for her technical assistance. This study was supported by a 2015 The University of California Institute for Mexico and the United States and El Consejo Nacional de Ciencia y Tecnología (UC MEXUS – CONACYT) collaborative research grant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guevara-Avendaño, E., Carrillo, J.D., Ndinga-Muniania, C. et al. Antifungal activity of avocado rhizobacteria against Fusarium euwallaceae and Graphium spp., associated with Euwallacea spp. nr. fornicatus, and Phytophthora cinnamomi . Antonie van Leeuwenhoek 111, 563–572 (2018). https://doi.org/10.1007/s10482-017-0977-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-017-0977-5