Abstract
The visual loss that occurs with sympathetic ophthalmia (SO) in the absence of recognizable retinal damage and inflammatory cell infiltration is an enigma. Experimental autoimmune uveoretinitis (EAU) is an animal model used to study human endogenous uveitis. Both innate and adaptive immune responses have been well studied in the photoreceptor damage mechanism of EAU. In our studies, in the early phase of EAU, proinflammatory molecules such as tumor necrosis factor (TNF)-α and inducible nitric oxide synthase (iNOS) and the subsequent mitochondrial DNA damage, mitochondrial protein alteration, and mitochondrial dysfunction by oxidative stress were observed before retinal inflammatory cell infiltration. Our recent study shows the importance of Toll-like receptors (TLRs) in the production of proinflammatory molecules and the induction of mitochondrial oxidative stress. Thus, the innate immune responses occur first with the activation of TLRs; this activation upregulates proinflammatory molecules, leading to mitochondrial oxidative stress before retinal inflammatory cell infiltration and the subsequent adaptive immune responses. Like EAU, SO also results in photoreceptor mitochondrial oxidative damage without retinal inflammatory cell infiltration. Such damage was associated with TNF-α, TNF-α receptors, and iNOS expression in the photoreceptors, suggesting that this molecular mechanism without retinal inflammatory cell infiltration may initiate photoreceptor damage in SO.



Similar content being viewed by others
References
Chan CC, Mochizuki M. Sympathetic ophthalmia: an autoimmune ocular inflammatory disease. Springer Semin Immunopathol. 1999;21:125–34.
Goto H, Rao NA. Sympathetic ophthalmia and Vogt–Koyanagi–Harada syndrome. Int Ophthalmol Clin. 1990;30:279–85.
Rao NA. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc. 1990;88:797–850.
Prieto-del-Cura M, González-Guijarro J. Complications of uveitis: prevalence and risk factors in a series of 398 cases. Arch Soc Esp Oftalmol. 2009;84:523–8.
Sarti P, Giuffrè A, Forte E, Mastronicola D, Barone MC, Brunori M. Nitric oxide and cytochrome C oxidase: mechanisms of inhibition and NO degradation. Biochem Biophys Res Commun. 2000;274:183–7.
Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, et al. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000;275:13175–8.
Carreras MC, Franco MC, Peralta JG, Poderoso JJ. Nitric oxide, complex I, and the modulation of mitochondrial reactive species in biology and disease. Mol Aspects Med. 2004;25:125–39.
Lisdero CL, Carreras MC, Meulemans A, Melani M, Aubier M, Boczkowski J, et al. The mitochondrial interplay of ubiquinol and nitric oxide in endotoxemia. Methods Enzymol. 2004;382:67–81.
Poderoso JJ, Carreras MC, Schöpfer F, Lisdero CL, Riobó NA, Giulivi C, et al. The reaction of nitric oxide with ubiquinol: kinetic properties and biological significance. Free Radic Biol Med. 1999;26:925–35.
Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1999;1411:351–69.
Brown GC, Borutaite V. Nitric oxide, cytochrome c and mitochondria. Biochem Soc Symp. 1999;66:17–25.
Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci. 2008;49:1161–71.
Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med. 2004;102:395–419.
Saraswathy S, Rao NA. Mitochondrial proteomics in experimental autoimmune uveitis oxidative stress. Invest Ophthalmol Vis Sci. 2009;50:5559–66.
Wu GS, Zhang J, Rao NA. Peroxynitrite and oxidative damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 1997;38:1333–9.
Zhang J, Wu LY, Wu GS, Rao NA. Differential expression of nitric oxide synthase in experimental uveoretinitis. Invest Ophthalmol Vis Sci. 1999;40:1899–905.
Rao NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Prog Retin Eye Res. 2000;19:41–68.
Rajendram R, Saraswathy S, Rao NA. Photoreceptor mitochondrial oxidative stress in early experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007;91:531–7.
Saraswathy S, Nguyen AM, Rao NA. The role of TLR4 in photoreceptor {alpha}a crystallin upregulation during early experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2010;51:3680–6.
Saraswathy S, Rao NA. Photoreceptor mitochondrial oxidative stress in experimental autoimmune uveitis. Ophthalmic Res. 2008;40:160–4.
Sartani G, Silver PB, Rizzo LV, Chan CC, Wiggert B, Mastorakos G, et al. Anti-tumor necrosis factor alpha therapy suppresses the induction of experimental autoimmune uveoretinitis in mice by inhibiting antigen priming. Invest Ophthalmol Vis Sci. 1996;37:2211–8.
Nakamura S, Yamakawa T, Sugita M, Kijima M, Ishioka M, Tanaka S, et al. The role of tumor necrosis factor-alpha in the induction of experimental uveoretinitis in mice. Invest Ophthalmol Vis Sci. 1994;35:3884–9.
Mizuguchi J, Takeuchi M, Usui M. Type I interferons as immunoregulatory molecules; implications for therapy in experimental autoimmune uveoretinitis. Arch Immunol Ther Exp. 2002;50:243–54.
Ooi KG, Galatowicz G, Towler HM, Lightman SL, Calder VL. Multiplex cytokine detection versus ELISA for aqueous humor: IL-5, IL-10, and IF gamma profiles in uveitis. Invest Ophthalmol Vis Sci. 2006;47:272–7.
Savion S, Oddo S, Grover S, Caspi RR. Lymphocytes in the rat: pathogenicity vs. lymphokine production, adhesion molecules and surface antigen expression. J Neuroimmunol. 1994;55:35–44.
Rao KM. Molecular mechanisms regulating iNOS expression in various cell types. J Toxicol Environ Health B Crit Rev. 2000;3:27–58.
Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13.
Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, et al. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA. 1993;90:3024–7.
Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–14.
Vásquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA Jr, Kalyanaraman B. Endothelial nitric oxide synthase dependent superoxide generation from adriamycin. Biochemistry. 1997;36:11293–7.
Valdez LB, Alvarez S, Arnaiz SL, Schöpfer F, Carreras MC, Poderoso JJ, et al. Reactions of peroxynitrite in the mitochondrial matrix. Free Radic Biol Med. 2000;29:349–56.
Calcerrada P, Peluffo G, Radi R. Nitric oxide-derived oxidants with a focus on peroxynitrite: molecular targets, cellular responses and therapeutic implications. Curr Pharm Des. 2011;17:3905–32.
Santos JH, Hunakova L, Chen Y, Bortner C, Van Houten B. Cell sorting experiments link persistent mitochondrial DNA damage with loss of mitochondrial membrane potential and apoptotic cell death. J Biol Chem. 2003;278:1728–34.
Mandavilli BS, Santos JH, Van Houten B. Mitochondrial DNA repair and aging. Mutat Res. 2002;509:127–51.
Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair (Amst). 2006;5:145–52.
Khurana RN, Parikh JG, Saraswathy S, Wu GS, Rao NA. Mitochondrial oxidative DNA damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2008;49:3299–304.
Wu GS, Lee TD, Moore RE, Rao NA. Photoreceptor mitochondrial tyrosine nitration in experimental uveitis. Invest Ophthalmol Vis Sci. 2005;46:2271–81.
Bornhövd C, Vogel F, Neupert W, Reichert AS. Mitochondrial membrane potential is dependent on the oligomeric state of F1F0–ATP synthase supracomplexes. J Biol Chem. 2006;281:13990–8.
Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol. 2004;40:861–8.
Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy. 2002;1:181–91.
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52.
Ohtaki H, Takeda T, Dohi K, Yofu S, Nakamachi T, Satoh K, et al. Increased mitochondrial DNA oxidative damage after transient middle cerebral artery occlusion in mice. Neurosci Res. 2007;58:349–55.
Ko MK, Saraswathy S, Parikh JG, Rao NA. The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Invest Ophthalmol Vis Sci. 2011;52:5824–35.
Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–9.
Albini TA, Wang RC, Reiser B, Zamir E, Wu GS, Rao NA. Microglial stability and repopulation in the retina. Br J Ophthalmol. 2005;89:901–3.
Santos AM, Martín-Oliva D, Ferrer-Martín RM, Tassi M, Calvente R, Sierra A, et al. Microglial response to light-induced photoreceptor degeneration in the mouse retina. J Comp Neurol. 2010;518:477–92.
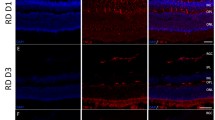
Parikh JG, Saraswathy S, Rao NA. Photoreceptor oxidative damage in sympathetic ophthalmia. Am J Ophthalmol. 2008;146:866–75.
Palexas GN, Sussman G, Welsh NH. Ocular and systemic determination of IL-1 beta and tumour necrosis factor in a patient with ocular inflammation. Scand J Immunol Suppl. 1992;11:173–5.
Tanel A, Averill-Bates DA. The aldehyde acrolein induces apoptosis via activation of the mitochondrial pathway. Biochim Biophys Acta. 2005;1743:255–67.
Goto H, Wu GS, Gritz DC, Atalla LR, Rao NA. Chemotactic activity of the peroxidized retinal membrane lipids in experimental autoimmune uveitis. Curr Eye Res. 1991;10:1009–14.
Lim ML, Lum MG, Hansen TM, Roucou X, Nagley P. On the release of cytochrome C from mitochondria during cell death signaling. J Biomed Sci. 2002;9:488–506.
Acknowledgments
This work was supported in part by The National Institute of Health grants EY017347, EY019506, and EY03040, and by a grant from Research to Prevent Blindness, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
The content of this invited review article was presented at the ARVO-JOS joint symposium on May 13, 2011, held during the 115th Annual Meeting of the Japanese Ophthalmological Society.
About this article
Cite this article
Kaneko, Y., Rao, N.A. Mitochondrial oxidative stress initiates visual loss in sympathetic ophthalmia. Jpn J Ophthalmol 56, 191–197 (2012). https://doi.org/10.1007/s10384-012-0132-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-012-0132-9