Abstract
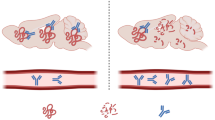
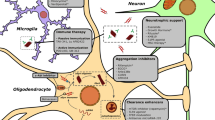
Multiple system atrophy (MSA) is a sporadic, adult onset, relentlessly progressive neurodegenerative disease characterized by autonomic abnormalities associated with parkinsonism, cerebellar dysfunction, pyramidal signs, or combinations thereof. Treatments that can halt or reverse the progression of MSA have not yet been identified. MSA is neuropathologically defined by the presence of α-synuclein-containing inclusions, particularly in the cytoplasm of oligodendrocytes (glial cytoplasmic inclusions, GCIs), which are associated with neurodegeneration. The mechanisms by which oligodendrocytic α-synuclein inclusions cause neuronal death in MSA are not completely understood. The MSA neurodegenerative process likely comprises cell-to-cell transmission of α-synuclein in a prion-like manner, α-synuclein aggregation, increased oxidative stress, abnormal expression of tubulin proteins, decreased expression of neurotrophic factors, excitotoxicity and microglial activation, and neuroinflammation. In an attempt to block each of these pathogenic mechanisms, several pharmacologic approaches have been tried and shown to exert neuroprotective effects in transgenic mouse or cellular models of MSA. These include sertraline, paroxetine, and lithium, which hamper arrival of α-synuclein to oligodendroglia; rifampicin, lithium, and non-steroidal anti-inflammatory drugs, which inhibit α-synuclein aggregation in oligodendrocytes; riluzole, rasagiline, fluoxetine and mesenchymal stem cells, which exert neuroprotective actions; and minocycline and intravenous immunoglobulins, which reduce neuroinflammation and microglial activation. These and other potential therapeutic strategies for MSA are summarized in this review.


Similar content being viewed by others
References
Wenning GK, Geser F, Krismer F et al (2013) The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12(3):264–274
Roncevic D, Palma JA, Martinez J, Goulding N, Norcliffe-Kaufmann L, Kaufmann H (2013) Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm 121(5):507–512
Watanabe H, Saito Y, Terao S et al (2002) Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 125(Pt 5):1070–1083
Kollensperger M, Geser F, Ndayisaba JP et al (2010) Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord 25(15):2604–2612
Iwai A, Masliah E, Yoshimoto M et al (1995) The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14(2):467–475
Jakes R, Spillantini MG, Goedert M (1994) Identification of two distinct synucleins from human brain. FEBS Lett 345(1):27–32
Lee FJ, Liu F, Pristupa ZB, Niznik HB (2001) Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J 15(6):916–926
Burre J, Vivona S, Diao J, Sharma M, Brunger AT, Sudhof TC (2013) Properties of native brain alpha-synuclein. Nature 498(7453):E4–E6 (discussion E6-7)
Bartels T, Choi JG, Selkoe DJ (2011) Alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477(7362):107–110
Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H (1998) Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett 249(2–3):180–182
Tu PH, Galvin JE, Baba M et al (1998) Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol 44(3):415–422
Tong J, Wong H, Guttman M et al (2010) Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: a comparative investigation. Brain 133(Pt 1):172–188
Wakabayashi K, Takahashi H (2006) Cellular pathology in multiple system atrophy. Neuropathology 26(4):338–345
Yoshida M (2007) Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology 27(5):484–493
Stefanova N, Reindl M, Neumann M et al (2005) Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol 166(3):869–876
Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB (2005) Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm 112(12):1613–1624
Ozawa T, Okuizumi K, Ikeuchi T, Wakabayashi K, Takahashi H, Tsuji S (2001) Analysis of the expression level of alpha-synuclein mRNA using postmortem brain samples from pathologically confirmed cases of multiple system atrophy. Acta Neuropathol 102(2):188–190
Lee SJ (2008) Origins and effects of extracellular alpha-synuclein: implications in Parkinson’s disease. J Mol Neurosci 34(1):17–22
Kordower JH, Freeman TB, Olanow CW (1998) Neuropathology of fetal nigral grafts in patients with Parkinson’s disease. Mov Disord 13(Suppl 1):88–95
Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ (2008) Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol 40(9):1835–1849
Tsigelny IF, Sharikov Y, Wrasidlo W et al (2012) Role of alpha-synuclein penetration into the membrane in the mechanisms of oligomer pore formation. FEBS J 279(6):1000–1013
Lee HJ, Bae EJ, Lee SJ (2014) Extracellular alpha-synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 10(2):92–98
Desplats P, Lee HJ, Bae EJ et al (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 106(31):13010–13015
Lee HJ, Suk JE, Patrick C et al (2010) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285(12):9262–9272
Hansen C, Angot E, Bergstrom AL et al (2011) Alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121(2):715–725
Masuda-Suzukake M, Nonaka T, Hosokawa M et al (2013) Prion-like spreading of pathological alpha-synuclein in brain. Brain 136(Pt 4):1128–1138
Watts JC, Giles K, Oehler A et al (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci USA 110(48):19555–19560
Chavarria C, Souza JM (2013) Oxidation and nitration of alpha-synuclein and their implications in neurodegenerative diseases. Arch Biochem Biophys 533(1–2):25–32
Giasson BI, Duda JE, Murray IV et al (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290(5493):985–989
Ubhi K, Lee PH, Adame A et al (2009) Mitochondrial inhibitor 3-nitropropionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res 87(12):2728–2739
Hanna PA, Jankovic J, Kirkpatrick JB (1999) Multiple system atrophy: the putative causative role of environmental toxins. Arch Neurol 56(1):90–94
Soma H, Yabe I, Takei A, Fujiki N, Yanagihara T, Sasaki H (2008) Associations between multiple system atrophy and polymorphisms of SLC1A4, SQSTM1, and EIF4EBP1 genes. Mov Disord 23(8):1161–1167
Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK (2012) Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res 21(4):393–404
Lindersson E, Lundvig D, Petersen C et al (2005) p25alpha Stimulates alpha-synuclein aggregation and is co-localized with aggregated alpha-synuclein in alpha-synucleinopathies. J Biol Chem 280(7):5703–5715
Song YJ, Lundvig DM, Huang Y et al (2007) p25alpha relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am J Pathol 171(4):1291–1303
Hasegawa T, Baba T, Kobayashi M et al (2010) Role of TPPP/p25 on alpha-synuclein-mediated oligodendroglial degeneration and the protective effect of SIRT2 inhibition in a cellular model of multiple system atrophy. Neurochem Int 57(8):857–866
Nakayama K, Suzuki Y, Yazawa I (2009) Microtubule depolymerization suppresses alpha-synuclein accumulation in a mouse model of multiple system atrophy. Am J Pathol 174(4):1471–1480
Goldbaum O, Jensen PH, Richter-Landsberg C (2008) The expression of tubulin polymerization promoting protein TPPP/p25alpha is developmentally regulated in cultured rat brain oligodendrocytes and affected by proteolytic stress. Glia 56(16):1736–1746
Stefanova N, Kaufmann WA, Humpel C, Poewe W, Wenning GK (2012) Systemic proteasome inhibition triggers neurodegeneration in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. Acta Neuropathol 124(1):51–65
Lee Y, Morrison BM, Li Y et al (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487(7408):443–448
Kawamoto Y, Nakamura S, Matsuo A, Akiguchi I (2000) Glial cell line-derived neurotrophic factor-like immunoreactivity in the cerebella of normal subjects and patients with multiple system atrophy. Acta Neuropathol 100(2):131–137
Ubhi K, Rockenstein E, Mante M et al (2010) Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci 30(18):6236–6246
Domercq M, Sanchez-Gomez MV, Sherwin C, Etxebarria E, Fern R, Matute C (2007) System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol 178(10):6549–6556
Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T (2004) Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol 63(1):43–52
Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK (2007) Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord 22(15):2196–2203
Gerhard A, Banati RB, Goerres GB et al (2003) [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology 61(5):686–689
Scherfler C, Sather T, Diguet E et al (2005) Riluzole improves motor deficits and attenuates loss of striatal neurons in a sequential double lesion rat model of striatonigral degeneration (Parkinson variant of multiple system atrophy). J Neural Transm 112(8):1025–1033
Bakiri Y, Burzomato V, Frugier G, Hamilton NB, Karadottir R, Attwell D (2009) Glutamatergic signaling in the brain’s white matter. Neuroscience 158(1):266–274
Fern R, Moller T (2000) Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J Neurosci 20(1):34–42
Butt AM (2006) Neurotransmitter-mediated calcium signalling in oligodendrocyte physiology and pathology. Glia 54(7):666–675
Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA (2005) Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA 102(28):9936–9941
Konno M, Hasegawa T, Baba T et al (2012) Suppression of dynamin GTPase decreases alpha-synuclein uptake by neuronal and oligodendroglial cells: a potent therapeutic target for synucleinopathy. Mol Neurodegener 7:38
Friess E, Kuempfel T, Modell S et al (2006) Paroxetine treatment improves motor symptoms in patients with multiple system atrophy. Parkinsonism Relat Disord 12(7):432–437
Ozawa T, Sekiya K, Sekine Y et al (2012) Maintaining glottic opening in multiple system atrophy: efficacy of serotonergic therapy. Mov Disord 27(7):919–921
Ubhi K, Rockenstein E, Mante M et al (2008) Rifampicin reduces alpha-synuclein in a transgenic mouse model of multiple system atrophy. NeuroReport 19(13):1271–1276
Nakayama K, Suzuki Y, Yazawa I (2012) Binding of neuronal alpha-synuclein to beta-III tubulin and accumulation in a model of multiple system atrophy. Biochem Biophys Res Commun 417(4):1170–1175
Low PA, Robertson D, Gilman S et al (2014) Efficacy and safety of rifampicin for multiple system atrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 13(3):268–275
Sacca F, Marsili A, Quarantelli M et al (2013) A randomized clinical trial of lithium in multiple system atrophy. J Neurol 260(2):458–461
Hirohata M, Ono K, Morinaga A, Yamada M (2008) Non-steroidal anti-inflammatory drugs have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. Neuropharmacology 54(3):620–627
El-Agnaf OM, Paleologou KE, Greer B et al (2004) A strategy for designing inhibitors of alpha-synuclein aggregation and toxicity as a novel treatment for Parkinson’s disease and related disorders. FASEB J 18(11):1315–1317
Kim YS, Lim D, Kim JY, Kang SJ, Kim YH, Im H (2009) beta-Sheet-breaking peptides inhibit the fibrillation of human alpha-synuclein. Biochem Biophys Res Commun 387(4):682–687
Shaltiel-Karyo R, Frenkel-Pinter M, Egoz-Matia N et al (2010) Inhibiting alpha-synuclein oligomerization by stable cell-penetrating beta-synuclein fragments recovers phenotype of Parkinson’s disease model flies. PLoS ONE 5(11):e13863
Mazzulli JR, Mishizen AJ, Giasson BI et al (2006) Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci 26(39):10068–10078
Shaltiel-Karyo R, Frenkel-Pinter M, Rockenstein E et al (2013) A blood–brain barrier (BBB) disrupter is also a potent alpha-synuclein (alpha-syn) aggregation inhibitor: a novel dual mechanism of mannitol for the treatment of Parkinson disease (PD). J Biol Chem 288(24):17579–17588
Di Giovanni S, Eleuteri S, Paleologou KE et al (2010) Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity. J Biol Chem 285(20):14941–14954
Shaltiel-Karyo R, Davidi D, Frenkel-Pinter M, Ovadia M, Segal D, Gazit E (2012) Differential inhibition of alpha-synuclein oligomeric and fibrillar assembly in Parkinson’s disease model by cinnamon extract. Biochim Biophys Acta 1820(10):1628–1635
Horvath I, Sellstedt M, Weise C et al (2013) Modulation of alpha-synuclein fibrillization by ring-fused 2-pyridones: templation and inhibition involve oligomers with different structure. Arch Biochem Biophys 532(2):84–90
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460(2):525–542
Cheah BC, Vucic S, Krishnan AV, Kiernan MC (2010) Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem 17(18):1942–1199
Bensimon G, Ludolph A, Agid Y et al (2009) Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain 132(Pt 1):156–171
Liu SB, Zhang N, Guo YY et al (2012) G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J Neurosci 32(14):4887–4900
Heo JH, Lee ST, Chu K, Kim M (2008) The efficacy of combined estrogen and buspirone treatment in olivopontocerebellar atrophy. J Neurol Sci 271(1–2):87–90
Alberdi E, Sanchez-Gomez MV, Torre I et al (2006) Activation of kainate receptors sensitizes oligodendrocytes to complement attack. J Neurosci 26(12):3220–3228
Stys PK, Lipton SA (2007) White matter NMDA receptors: an unexpected new therapeutic target? Trends Pharmacol Sci 28(11):561–566
Matute C (2008) P2X7 receptors in oligodendrocytes: a novel target for neuroprotection. Mol Neurobiol 38(2):123–128
Olanow CW, Rascol O, Hauser R et al (2009) A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 361(13):1268–1278
Jenner P, Langston JW (2011) Explaining ADAGIO: a critical review of the biological basis for the clinical effects of rasagiline. Mov Disord 26(13):2316–2323
Stefanova N, Poewe W, Wenning GK (2008) Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol 210(2):421–427
ClinicalTrials.gov Identifier: NCT00977665
Holmberg B, Johansson JO, Poewe W et al (2007) Safety and tolerability of growth hormone therapy in multiple system atrophy: a double-blind, placebo-controlled study. Mov Disord 22(8):1138–1144
Ubhi K, Inglis C, Mante M et al (2012) Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of alpha-synucleinopathy. Exp Neurol 234(2):405–416
Zhang F, Zhou H, Wilson BC, Shi JS, Hong JS, Gao HM (2012) Fluoxetine protects neurons against microglial activation-mediated neurotoxicity. Parkinsonism Relat Disord 18(Suppl 1):S213–S217
Kohl Z, Winner B, Ubhi K et al (2012) Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci 35(1):10–19
ClinicalTrials.gov Identifier: NCT01146548
Dezawa M, Kanno H, Hoshino M et al (2004) Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest 113(12):1701–1710
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98(5):1076–1084
Park HJ, Bang G, Lee BR, Kim HO, Lee PH (2011) Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy Parkinsonism. Cell Transpl 20(6):827–835
Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK (2011) Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS ONE 6(5):e19808
Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K (2008) Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther 83(5):723–730
Quinn N, Barker RA, Wenning GK (2008) Are trials of intravascular infusions of autologous mesenchymal stem cells in patients with multiple system atrophy currently justified, and are they effective? Clin Pharmacol Ther 83(5):663–665
Lee PH, Lee JE, Kim HS et al (2012) A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol 72(1):32–40
Low PA, Gilman S (2012) Are trials of intravascular infusions of autologous mesenchymal stem cells in patients with multiple system atrophy currently justified, and are they effective? Ann Neurol 72(1):4–5
Benarroch EE (2013) Microglia: multiple roles in surveillance, circuit shaping, and response to injury. Neurology 81(12):1079–1088
Dheen ST, Kaur C, Ling EA (2007) Microglial activation and its implications in the brain diseases. Curr Med Chem 14(11):1189–1197
Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID (2007) Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem 282(20):15208–15216
Dodel R, Spottke A, Gerhard A et al (2010) Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial). Mov Disord 25(1):97–107
Patwa HS, Chaudhry V, Katzberg H, Rae-Grant AD, So YT (2012) Evidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 78(13):1009–1015
Novak P, Williams A, Ravin P, Zurkiya O, Abduljalil A, Novak V (2012) Treatment of multiple system atrophy using intravenous immunoglobulin. BMC Neurol 12:131
Patrias LM, Klaver AC, Coffey MP, Loeffler DA (2010) Specific antibodies to soluble alpha-synuclein conformations in intravenous immunoglobulin preparations. Clin Exp Immunol 161(3):527–535
Masliah E, Rockenstein E, Mante M et al (2011) Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE 6(4):e19338
Bae EJ, Lee HJ, Rockenstein E et al (2012) Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J Neurosci 32(39):13454–13469
http://www.sympath-project.eu/. Accessed May 17 2014
Acknowledgments
HK receives research support from the National Institutes of Health (NIH) U54NS065736. The Autonomic Disorders Consortium (U54NS065736) is a part of the NIH Rare Diseases Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the NINDS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. JAP receives support from The Dysautonomia Foundation, Inc. We acknowledge The Multiple System Atrophy (MSA) Coalition for supporting the 24th International Symposium on the Autonomic Nervous System where this paper was initially presented.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palma, JA., Kaufmann, H. Novel therapeutic approaches in multiple system atrophy. Clin Auton Res 25, 37–45 (2015). https://doi.org/10.1007/s10286-014-0249-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-014-0249-7