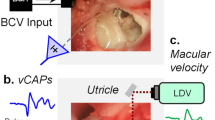
Abstract
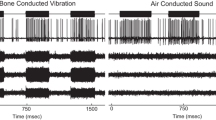
To explore the effects of cochlear hair cell displacement, researchers have previously monitored functional and mechanical responses during low-frequency (LF) acoustic stimulation of the cochlea. The induced changes are believed to result from modulation of the conductance of mechano-electrical transduction (MET) channels on cochlear hair cells, along with receptor potential modulation. It is less clear how, or if, vestibular hair cell displacement affects vestibular function. Here, we have used LF (<20 Hz) hydrodynamic modulation of the utricular macula position, whilst recording functional and mechanical responses, to investigate the effects of utricular macula displacement. Measured responses included the Utricular Microphonic (UM), the vestibular short-latency evoked potential (VsEP), and laser Doppler vibrometry recordings of macular position. Over 1 cycle of the LF bias, the UM amplitude and waveform were cyclically modulated, with Boltzmann analysis suggesting a cyclic modulation of the vestibular MET gating. The VsEP amplitude was cyclically modulated throughout the LF bias, demonstrating a relative increase (~20–50 %; re baseline) and decrease (~10–20 %; re baseline), which is believed to be related to the MET conductance and vestibular hair cell sensitivity. The relationship between macular displacement and changes in UM and VsEP responses was consistent within and across animals. These results suggest that the sensory structures underlying the VsEP, often thought to be a cranial jerk-sensitive response, are at least partially sensitive to LF (and possibly static) pressures or motion. Furthermore, these results highlight the possibility that some of the vestibular dysfunction related to endolymphatic hydrops may be due to altered vestibular transduction following mechanical (or morphological) changes in the labyrinth.










Similar content being viewed by others
References
Bian L, Watts KL (2008) Effects of low-frequency biasing on spontaneous otoacoustic emissions: amplitude modulation. J Acoust Soc Am 123:887–898
Bohmer A (1993) Hydrostatic pressure in the inner ear fluid compartments and its effects on inner ear function. Acta Otolaryngol Suppl 507:3–24
Brown DJ, Gibson WP (2011) On the differential diagnosis of Meniere's disease using low-frequency acoustic biasing of the 2f1-f2 DPOAE. Hear Res 282:119–127
Brown DJ, Hartsock JJ, Gill RM, Fitzgerald HE, Salt AN (2009) Estimating the operating point of the cochlear transducer using low-frequency biased distortion products. J Acoust Soc Am 125:2129–2145
Brown D, Chihara Y, Wang Y (2013a) Changes in utricular function during artificial endolymph injections in guinea pigs. Hear Res 304:70–76
Brown DJ, Chihara Y, Curthoys IS, Wang Y, Bos M (2013b) Changes in cochlear function during acute endolymphatic hydrops development in guinea pigs. Hear Res 296:96–106
Brown DJ, Sokolic L, Fung A, Pastras CJ (2018) Response of the inner ear to lipopolysaccharide introduced directly into scala media. Hear Res 370:105–112
Cheatham MA, Dallos P (1994) Stimulus biasing: a comparison between cochlear hair cell and organ of Corti response patterns. Hear Res 75:103–113
Deatherage BH, Henderson D (1967) Auditory sensitization. The Journal of the Acoustical Society of America 42:438–440
Dlugaiczyk J, Burgess AM, Goonetilleke SC, Sokolic L, Curthoys IS (2019) Superior canal dehiscence syndrome: relating clinical findings with vestibular neural responses from a guinea pig model. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 40:e406–e414
Elidan J, Lin J, Honrubia V (1986) The effect of loop diuretics on the vestibular system. Assessment by recording the vestibular evoked response Archives of otolaryngology--head & neck surgery 112:836–839
Flock Å, Flock B (2003) Micro-lesions in Reissner’s membrane evoked by acute hydrops. Audiology and Neurotology 8:59–69
Foster CA, Breeze RE (2013) The Meniere attack: an ischemia/reperfusion disorder of inner ear sensory tissues. Med Hypotheses 81:1108–1115
Guinan JJ Jr (2012) How are inner hair cells stimulated? Evidence for multiple mechanical drives. Hear Res 292:35–50
Holton T, Hudspeth AJ (1986) The transduction channel of hair cells from the bull-frog characterized by noise analysis. J Physiol 375:195–227
Jones TA, Jones SM, Vijayakumar S, Brugeaud A, Bothwell M, Chabbert C (2011) The adequate stimulus for mammalian linear vestibular evoked potentials (VsEPs). Hear Res 280:133–140
Kingma CM, Wit HP (2010) The effect of changes in perilymphatic K+ on the vestibular evoked potential in the guinea pig. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery 267:1679–1684
Kirk DL, Patuzzi RB (1997) Transient changes in cochlear potentials and DPOAEs after low-frequency tones: the 'two-minute bounce' revisited. Hear Res 112:49–68
Klis JF, Smoorenburg GF (1985) Modulation at the guinea pig round window of summating potentials and compound action potentials by low-frequency sound. Hear Res 20:15–23
Klis JF, Smoorenburg GF (1988) Cochlear potentials and their modulation by low-frequency sound in early endolymphatic hydrops. Hear Res 32:175–184
Li A, Xue J, Peterson EH (2008) Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol 99:718–733
Lichtenhan JT (2012) Effects of low-frequency biasing on otoacoustic and neural measures suggest that stimulus-frequency otoacoustic emissions originate near the peak region of the traveling wave. J Assoc Res Otolaryngol 13:17–28
Manzari L, Tedesco AR, Burgess AM, Curthoys IS (2010) Ocular and cervical vestibular-evoked myogenic potentials to bone conducted vibration in Meniere's disease during quiescence vs during acute attacks. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 121:1092–1101
McGarvie LA, Curthoys IS, MacDougall HG, Halmagyi GM (2015) What does the dissociation between the results of video head impulse versus caloric testing reveal about the vestibular dysfunction in Ménière’s disease? Acta Otolaryngol 135:859–865
Minor LB (2005) Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope 115:1717–1727
Nam JH, Grant JW, Rowe MH, Peterson EH (2019) Multiscale modeling of mechanotransduction in the utricle. J Neurophysiol 122:132–150
Nieder P, Nieder I (1968a) Some effects of tonal interactions as seen in the cochlear microphonic. The Journal of the Acoustical Society of America 43:1092–1106
Nieder P, Nieder I (1968b) Studies of two-tone interaction as seen in the guinea pig microphonic. The Journal of the Acoustical Society of America 44:1409–1422
Pastras CJ (2018) Assessment of utricular nerve, Hair Cell and Mechanical function, in vivo
Pastras C, Curthoys I, Brown D (2017) In vivo recording of the vestibular microphonic in mammals. Hear Res 354:38–47
Pastras CJ, Curthoys IS, Brown DJ (2018a) Dynamic response to sound and vibration of the guinea pig utricular macula, measured in vivo using laser Doppler Vibrometry. Hear Res 370:232–237
Pastras CJ, Curthoys IS, Sokolic L, Brown DJ (2018b) Suppression of the vestibular short-latency evoked potential by electrical stimulation of the central vestibular system. Hear Res 361:23–35
Patuzzi RB (1987) A model of the generation of the cochlear microphonic with nonlinear hair cell transduction and nonlinear basilar membrane mechanics. Hear Res 30:73–82
Patuzzi R (2011) Ion flow in cochlear hair cells and the regulation of hearing sensitivity. Hear Res 280:3–20
Patuzzi R, Moleirinho A (1998) Automatic monitoring of mechano-electrical transduction in the guinea pig cochlea. Hear Res 125:1–16
Patuzzi R, Sellick PM (1983a) The alteration of the low frequency response of primary auditory afferents by cochlear trauma. Hear Res 11:125–132
Patuzzi R, Sellick PM (1983b) A comparison between basilar membrane and inner hair cell receptor potential input-output functions in the guinea pig cochlea. J Acoust Soc Am 74:1734–1741
Patuzzi R, Sellick P (1984) The modulation of the sensitivity of the mammalian cochlea by low frequency tones II. Inner hair cell receptor potentials. Hearing research 13:9–18
Patuzzi RB, Yates GK (1987) The low-frequency response of inner hair cells in the guinea pig cochlea: implications for fluid coupling and resonance of the stereocilia. Hear Res 30:83–98
Patuzzi R, Sellick P, Johnstone B (1984a) The modulation of the sensitivity of the mammalian cochlea by low frequency tones. III. Basilar membrane motion. Hearing research 13:19–27
Patuzzi R, Sellick PM, Johnstone BM (1984b) The modulation of the sensitivity of the mammalian cochlea by low frequency tones. I. Primary afferent activity. Hear Res 13:1–8
Peng AW, Effertz T, Ricci AJ (2013) Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron 80:960–972
Pierson M, Møller A (1980) Effect of modulation of basilar membrane position on the cochlear microphonic. Hear Res 2:151–162
Rabbitt R, Yamauchi A, Boyle R, Highstein S (2001) How endolymph pressure modulates semicircular canal primary afferent discharge. Ann N Y Acad Sci 942:313–321
Rhode WS, Cooper NP (1993) Two-tone suppression and distortion production on the basilar membrane in the hook region of cat and guinea pig cochleae. Hear Res 66:31–45
Rosowski JJ, Songer JE, Nakajima HH, Brinsko KM, Merchant SN (2004) Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otology & Neurotology 25:323–332
Russell IJ, Kössl M (1992) Modulation of hair cell voltage responses to tones by low-frequency biasing of the basilar membrane in the guinea pig cochlea. J Neurosci 12:1587–1601
Sachs MB, Hubbard AE (1981) Responses of auditory-nerve fibers to characteristic-frequency tones and low-frequency suppressors. Hear Res 4:309–324
Salt AN, Plontke SK (2010) Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngol Clin N Am 43:971–983
Salt AN, Brown DJ, Hartsock JJ, Plontke SK (2009) Displacements of the organ of Corti by gel injections into the cochlear apex. Hear Res 250:63–75
Salt AN, Lichtenhan JT, Gill RM, Hartsock JJ (2013) Large endolymphatic potentials from low-frequency and infrasonic tones in the guinea pig. J Acoust Soc Am 133:1561–1571
Schuknecht H (1976) Pathophysiology of endolymphatic hydrops. Archives of oto-rhino-laryngology 212:253–262
Sellick P, Patuzzi R, Johnstone B (1982) Modulation of responses of spiral ganglion cells in the guinea pig cochlea by low frequency sound. Hear Res 7:199–221
Sirjani DB, Salt AN, Gill RM, Hale SA (2004) The influence of transducer operating point on distortion generation in the cochlea. J Acoust Soc Am 115:1219–1229
Songer JE, Rosowski JJ (2005) The effect of superior canal dehiscence on cochlear potential in response to air-conducted stimuli in chinchilla. Hear Res 210:53–62
Songer JE, Rosowski JJ (2006) The effect of superior-canal opening on middle-ear input admittance and air-conducted stapes velocity in chinchilla. The Journal of the Acoustical Society of America 120:258–269
Spoon C, Moravec WJ, Rowe MH, Grant JW, Peterson EH (2011) Steady-state stiffness of utricular hair cells depends on macular location and hair bundle structure. J Neurophysiol 106:2950–2963
Tonndorf J (1957) The mechanism of hearing loss in early cases of endolymphatic hydrops. The Annals of otology, rhinology, and laryngology 66:766–784
Tono T, Morizono T (1995) Low-frequency biasing of round window responses in guinea pigs and chinchillas. Audiology : official organ of the International Society of Audiology 34:47–56
Vollrath MA, Eatock RA (2003) Time course and extent of mechanotransducer adaptation in mouse utricular hair cells: comparison with frog saccular hair cells. J Neurophysiol 90:2676–2689
Vries HD, Bleeker JD (1949) The microphonic activity of the labyrinth of the pigeon: part II: the response of the cristae in the semicircular canals. Acta Otolaryngol 37:298–306
Wen MH, Cheng PW, Young YH (2012) Augmentation of ocular vestibular-evoked myogenic potentials via bone-conducted vibration stimuli in Meniere disease. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 146:797–803
Wit H, Tideman B, Segenhout J (1988) Modulation of microphonics: a new method to study the vestibular system. In: clinical testing of the vestibular system. Karger publishers, pp 59–64
Wu YC, Ricci AJ, Fettiplace R (1999) Two components of transducer adaptation in auditory hair cells. J Neurophysiol 82:2171–2181
Xue J, Peterson EH (2006) Hair bundle heights in the utricle: differences between macular locations and hair cell types. J Neurophysiol 95:171–186
Young ED, Fernández C, Goldberg JM (1977) Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol 84:352–360
Zhang J, Chen S, Hou Z, Cai J, Dong M, Shi X (2015) Lipopolysaccharide-induced middle ear inflammation disrupts the cochlear intra-strial fluid-blood barrier through down-regulation of tight junction proteins. PLoS One 10:e0122572
Zhou W, Nam J-H (2019) Probing hair cell’s mechano-transduction using two-tone suppression measurements. Sci Rep 9:4626
Zwicker E (1977) Masking-period patterns produced by very-low-frequency maskers and their possible relation to basilar-membrane displacement. The Journal of the Acoustical Society of America 61:1031–1040
Acknowledgements
This work was supported by the Sydney Medical School Foundation at The University of Sydney, with funds raised by the Meniere’s Research Fund Inc., and the Garnett Passe and Rodney Williams Memorial Foundation with support through a Junior Fellowship Grant. We thank Dr. Densil Cabrera and Mr. Jonothan Holmes from the University of Sydney School of Architecture, Design and Planning for supplying the Ometron Laser Doppler Vibrometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pastras, C.J., Stefani, S.P., Curthoys, I.S. et al. Utricular Sensitivity during Hydrodynamic Displacements of the Macula. JARO 21, 409–423 (2020). https://doi.org/10.1007/s10162-020-00769-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-020-00769-w