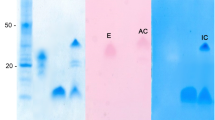
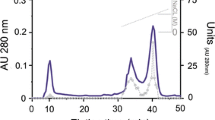
Abstract
A chymotrypsin was purified from the gastric juice of California spiny lobster (Panulirus interrutpus), using preparative electrophoresis and affinity chromatography on agarose-p-aminobenzamidine. The molecular mass was estimated by polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions to be 28 kDa. Chymotrypsin activity was totally inhibited by phenylmethylsulfonyl fluoride (PMSF) and chymostatin. Lobster chymotrypsin had optimal pH 7.0–8.0 and temperature of 55 °C. The enzyme is highly stable under a wide range of pH (retaining up to 80 % of activity after 1 h of incubation at pH 3.0, 5.0, and 12.0), showing higher stability at pH 8.0, and was inactivated after 20 min at 55 °C. Lobster chymotrypsin was able to hydrolyze protein substrates at as low as pH 3.0. These results are consistent with the findings of enzyme stability. Activity was assessed after incubation of enzyme with different organic solvents (in the range of 10–50 %); when tested in the presence of acetone, ethanol, propanol, and butanol, lobster chymotrypsin residual activity was >80 %; whereas in the presence of dimethyl sulfoxide (DMSO) and toluene, lobster chymotrypsin residual activity was <80 %. Deduced amino acid sequence, corroborated by mass spectrometry, was determined.







Similar content being viewed by others
References
Balti R, Bougherra F, Bougatef A, Hayet BK, Nedjar-Arroume N, Dhulster P, Guillochon D, Nasri M (2012) Chymotrypsin from the hepatopancreas of cuttlefish (Sepia officinalis) with high activity in the hydrolysis of long chain peptide substrates: purification and biochemical characterisation. Food Chem 130:475–484
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castillo-Yañez FJ, Pacheco-Aguilar R, Garcia-Carreño FL, Navarrete-Del Toro MDLA (2004) Characterization of acidic proteolytic enzymes from Monterey sardine (Sardinops sagax caerulea) viscera. Food Chem 85:343–350
Castillo-Yáñez FJ, Pacheco-Aguilar R, García-Carreño FL, Navarrete-Del Toro MA, Félix López M (2006) Purification and biochemical characterization of chymotrypsin from the viscera of Monterey sardine (Sardinops sagax caeruleus). Food Chem 99:252–259
Castillo-Yañez FJ, Pacheco-Aguilar R, Lugo-Sanchez ME, Garcia-Sanchez G, Quintero Reyes IE (2009) Biochemical characterization of an isoform of chymotrypsin from the viscera of Monterey sardine (Sardinops sagax caerulea), and comparison with bovine chymotrypsin. Food Chem 112:634–639
Celis-Guerrero LE, García-Carreño FL, Navarrete Del Toro MA (2004) Characterization of proteases in the digestive system of spiny lobster (Panulirus interruptus). Mar Biotechnol 6:262–269
D’Amico S, Claverie P, Collins T, Georlette D, Gratia E, Hoyoux A, Meuwis M-A, Feller G, Gerday C (2002) Molecular basis of cold adaptation. Philos Trans R Soc Lond B Biol Sci 357:917–925
DelMar EG, Largman C, Brodrick JW, Geokas MC (1979) A sensitive new substrate for chymotrypsin. Anal Biochem 99:316–320
Di Cera E (2009) Serine proteases. IUBMB Life 61:510–515
Díaz-Tenorio LM, García-Carreño FL, Navarrete del Toro MA (2006) Characterization and comparison of digestive proteinases of the Cortez swimming crab, Callinectes bellicosus, and the arched swimming crab, Callinectes arcuatus. Invertebr Biol 125:125–135
Eberhardt J (1992) Isolation and characterization of 5 serine proteases with trypsin-like, chymotrypsin-like and elastase-like characteristics from the gut of the lugworm Arenicola marina (L.) (Polychaeta). J Comp Physiol B 162:159–167
El Hadj AN, Hmidet N, Zouari-Fakhfakh N, Ben Khaled H, Nasri M (2010) Alkaline chymotrypsin from striped seabream (Lithognathus mormyrus) viscera: purification and characterization. J Agric Food Chem 58:9787–9792
Fong WP, Chan EY, Lau KK (1998) Isolation of two chymotrypsins from grass carp. Biochem Mol Biol Int 45:409–418
García-Carreño F, Dimes L, Haard N (1993) Substrate-gel electrophoresis for composition of molecular weight of proteinases or protteinaceous proteinase inhibitors. Anal Biochem 214:65–69
Geok LP, Razak CNA, Abd Rahman RNZ, Basri M, Salleh AB (2003) Isolation and screening of an extracellular organic solvent-tolerant protease producer. Biochem Eng J 13:73–77
Gráf L, Szilágyi L, Venekei I (2013) Chymotrypsin. In: Rawlings ND, Salvesen G (eds) Handbook of Proteolytic Enzymes, 3rd edn. Academic Press, San Diego, p 2626–2633
Gupta MN (1992) Enzyme function in organic solvents. Eur J Biochem 203:25–32
Hedstrom L (2002) Serine protease mechanism and specificity. Chem Rev 102:4501–4523
Hernández-Cortés P, Whitaker JR, García-Carreño F (1997) Purification and characterization of chymotrypsin from Penaeus vannamei. J Food Biochem 21:497–514
Heu MS, Kim HR, Pyeun JH (1995) Comparison of trypsin and chymotrypsin from the viscera of anchovy, Engraulis japonica. Comp Biochem Physiol B: Biochem Mol Biol 112:557–567
Klomklao S (2008) Digestive proteinases from marine organisms and their applications. Songklanakarin J Sci Technol 30:37–46
Kristjansson MM (1991) Purification and characterization of trypsin from the pyloric ceca of rainbow trout (Oncorhynchus mykiss). J Agric Food Chem 39:1738–1742
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Le Chevalier P, Sellos D, Van Wormhoudt A (1995) Purification and partial characterization of chymotrypsin-like proteases from the digestive gland of the scallop Pecten maximus. Comp Biochem Physiol B Biochem Mol Biol 110:777–784
Li GY, Pukunaga S, Takenouchi K, Nakamura F (2005) Comparative study of the physiological properties of collagen, gelatin and collagen hydrolysate as cosmetic materials. Int J Cosmet Sci 27:101–106
Lima CA, Rodrigues PMB, Porto TS, Viana DA, Lima Fihlo JL, Porto ALF, Carneiro da Cunha MG (2009) Production of a collagenase from Candida albicans URM3622. Biochem Eng J 43:315–320
Mares-Guia M, Shaw E (1965) Studies on the Active Center of Trypsin: the binding of amidines and guanidines as models of the substrate side chain. J Biol Chem 240:1579–1585
Muhlia-Almazán A, García-Carreño FL (2002) Influence of molting and starvation on the synthesis of proteolytic enzymes in the midgut gland of the white shrimp Penaeus vannamei. Comp Biochem Physiol B Biochem Mol Biol 133:383–394
Muhlia-Almazán A, Sánchez-Paz A, García-Carreño FL (2008) Invertebrate trypsins: a review. J Comp Physiol B 178:655–672
Navarrete del Toro MDLA, García-Carreño F, López MD, Celis-Guerrero L, Saborowski R (2006) Aspartic proteinases in the digestive tract of marine decapod crustaceans. J Exp Zool A Comp Exp Biol 305:645–654
Navarrete del Toro MA, García-Carreño FL, Córdova-Murueta JH (2011) Comparison of digestive proteinases in three penaeids. Aquaculture 317:99–106
Navarrete-del-Toro MA, García-Carreño Fernando L, Hernández-Cortés P, Molnár T, Gráf L (2015) Biochemical characterisation of chymotrypsin from the midgut gland of yellowleg shrimp, Penaeus Californiensis. Food Chem 173:147–155
Neurath H (1957) The activation of zymogens. In: Anfisen CB, Anson ML, Bailey K, Edsall JT (eds) Advances in Protein Chemistry. Academic Press, New York, p 319–386
Perera E, Fraga I, Carrillo O, Díaz-Iglesias E, Cruz R, Báez M, Galich GS (2005) Evaluation of practical diets for the Caribbean spiny lobster Panulirus argus (Latreille, 1804): effects of protein sources on substrate metabolism and digestive proteases. Aquaculture 244:251–262
Perera E, Moyano FJ, Díaz M, Perdomo-Morales R, Montero-Alejo V, Carrillo O, Galich GS (2008a) Polymorphism and partial characterization of digestive enzymes in the spiny lobster Panulirus argus. Comp Biochem Physiol B Biochem Mol Biol 150:247–254
Perera E, Moyano FJ, Díaz M, Perdomo-Morales R, Montero-Alejo V, Rodriguez-Viera L, Alonso E, Carrillo O, Galich GS (2008b) Changes in digestive enzymes through developmental and molt stages in the spiny lobster, Panulirus argus. Comp Biochem Physiol B Biochem Mol Biol 151:250–256
Perona JJ, Craik CS (1995) Structural basis of substrate specificity in the serine proteases. Protein Sci 4:337–360
Perona JJ, Craik CS (1997) Evolutionary divergence of substrate specificity within the chymotrypsin-like serine protease fold. J Biol Chem 272:29987–29990
Raae AJ, Walther BT (1989) Purification and characterization of chymotrypsin, trypsin and elastase like proteinases from cod (Gadus morhua L.). Comp Biochem Physiol B 93:317–324
Rojo L, Muhlia-Almazan A, Saborowski R, García-Carreño F (2010a) Aspartic cathepsin D endopeptidase contributes to extracellular digestion in clawed lobsters Homarus americanus and Homarus gammarus. Mar Biotechnol 12:696–707
Rojo L, Sotelo-Mundo R, García-Carreño F, Gráf L (2010b) Isolation, biochemical characterization, and molecular modeling of American lobster digestive cathepsin D1. Comp Biochem Physiol B Biochem Mol Biol 157:394–400
Roy P, Colas B, Durand P (1996) Purification, kinetical and molecular characterizations of a serine collagenolytic protease from greenshore crab (Carcinus maenas) digestive gland. Comp Biochem Physiol B Biochem Mol Biol 115:87–95
Rudenskaya GN (2003) Brachyurins, serine collagenolytic enzymes from crabs. Russ J Bioorg Chem 29:101–111
Saborowski R, Sahling G, Navarrete del Toro MA, Walter I, García-Carreño FL (2004) Stability and effects of organic solvents on endopeptidases from the gastric fluid of the marine crab Cancer pagurus. J Mol Catal B Enzym 30:109–118
Shi XZ, Zhao XF, Wang JX (2008) Molecular cloning and expression analysis of chymotrypsin-like serine protease from the Chinese shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol 25:589–597
Stauffer C (1989) Enzyme assays for food scientist. Chapman and Hall, New York, p 61–76
Teschke M, Saborowski R (2005) Cysteine proteinases substitute for serine proteinases in the midgut glands of Crangon crangon and Crangon allmani (Decapoda: Caridea). J Exp Mar Biol Ecol 316:213–229
Tsai I-H, Chuano K-L, Chuang JL (1986) Chymotrypsins in digestive tracts of crustacean decapods (Shrimps). Comp Biochem Physiol B 85:235–239
Tsai IH, Lu PJ, Chuang JL (1991) The midgut chymotrypsins of shrimps (Penaeus monodon, Penaeus japonicus and Penaeus penicillatus). Biochim Biophys Acta 1080:59–67
Tsu CA, Perona JJ, Schellenberger V, Turck CW, Craik CS (1994) The substrate specificity of Uca pugilator collagenolytic serine protease 1 correlates with the bovine type I collagen cleavage sites. J Biol Chem 269:19565–19572
Van Wormhoudt A, Le Chevalier P, Sellos D (1992) Purification, biochemical characterization and N-terminal sequence of a serine-protease with chymotrypsic and collagenolytic activities in a tropical shrimp, Penaeus vannamei (Crustacea, Decapoda). Comp Biochem Physiol B 103:675–680
Yang F, Su W-J, Lu B-J, Wu T, Sun L-C, Hara K, Cao M-J (2009) Purification and characterization of chymotrypsins from the hepatopancreas of crucian carp (Carassius auratus). Food Chem 116:860–866
Acknowledgments
We thank Patricia Hernandez-Cortes for technical assistance and Ira Fogel for comprehensive editing services (CIBNOR). This study was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT grant 80935 to F.G.C.). B.B.V. is a recipient of a graduate fellowship (CONACYT 277859). We thank Dr. Reinhard Saborowski at AWI, Germany, for supporting this research.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bibo-Verdugo, B., Rojo-Arreola, L., Navarrete-del-Toro, M.A. et al. A chymotrypsin from the Digestive Tract of California Spiny Lobster, Panulirus interruptus: Purification and Biochemical Characterization. Mar Biotechnol 17, 416–427 (2015). https://doi.org/10.1007/s10126-015-9626-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-015-9626-z