Abstract
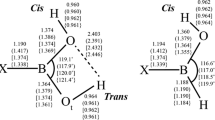
In this work, the spectroscopic information, stability and aromaticity of the boron-nitrogen azulene and naphthalene molecules are provided by the use of CC2 (geometry optimization, dipole moment, UV–vis spectrum calculations) and DFT (vibrational spectrum and NMR calculations) methodologies. One isomer of the investigated boron-nitrogen naphthalene (boroazanaphthalene) and two isomers of boron-nitrogen azulene, 1,3,4,6,8-pentaaza-2,3a,5,7,8a-pentaboraazulene (BN-azulene) and 2,3a,5,7,8a-pentaaza-1,3,4,6,8- pentaboraazulene (NB-azulene), are stable systems. However, these molecules have different properties, i.e., different stability, dipole moment, and aromaticity based on the NICS approach. BN-naphthalene has a high dipole moment magnitude showing high polar character, while naphthalene is apolar. BN- and NB-azulene are weakly polar, while ordinary azulene is highly polar in character. Also, substitution of C atoms by B and N atoms decreases the aromaticity. In the case of NB-azulene, the seven-membered ring has anti-aromaticity behavior while both rings of BN-azulene exhibit aromaticity. We expect that the new theoretical data provided in this work will be useful in identifying and characterizing experimentally the compounds investigated, and in helping our understanding of the chemistry of boron-nitrogen molecules.

Boron-nitrogen alternating analogs of azulene. Spectral distinction between isomers






Similar content being viewed by others
References
Campbell PG, Marwitz AJ, Liu SY (2012) Recent advances in azaborine chemistry. Angew Chem Int Ed Engl 51(25):6074–6092. doi:10.1002/anie.201200063
Kesharwani MK, Suresh M, Das A, Ganguly B (2011) Borazine as a sensor for fluoride ion: a computational and experimental study. Tetrahedron Lett 52(28):3636–3639. doi:10.1016/j.tetlet.2011.05.018
Song Y, Zhang C, Li B, Ding G, Jiang D, Wang H, Xie X (2014) Van der Waals epitaxy and characterization of hexagonal boron nitride nanosheets on graphene. Nanoscale Res Lett 9:367–373
J-s L, C-r Z, Li B, Cao F, Wang S-q (2011) An investigation on the synthesis of borazine. Inorg Chim Acta 366(1):173–176. doi:10.1016/j.ica.2010.10.030
Lisovenko AS, Timoshkin AY (2010) Donor-acceptor complexes of borazines. Inorg Chem 49(22):10357–10369. doi:10.1021/ic101081k
de Abreu L, Lopez-Castillo A (2012) Theoretical characterization of the BN and BP coronenes by IR, Raman, and UV–VIS spectra. J Chem Phys 137(4):044309–044305. doi:10.1063/1.4737519
Rad AS, Ayub K (2016) Enhancement in hydrogen molecule adsorption on B12N12 nano-cluster by decoration of nickel. Int J Hydrog Energy 41(47):22182–22191. doi:10.1016/j.ijhydene.2016.08.158
Weinmann M, Seifert HJ, Aldinger F (2000) Boron-containing, non-oxide ceramics from organometallic polymers: synthesis, thermolysis and the influence of boron on materials thermal stability. In: Davidson M, Hughes AK, Marder TB, Wade K (eds) Contemporary boron chemistry. The Royal Society of Chemistry, UK, pp 88–91
Golberg D, Bando Y, Kurashima K, Sato T (2001) Synthesis and characterization of ropes made of bn multiwalled nanotubes. Scr Mater 44:1561–1565
Ishii T, Sato T, Sekikawa Y, Iwata M (1981) Growth of whiskers of hexagonal boron nitride. J Cryst Growth 52:285–289
Lin Y, Williams TV, Connell JW (2009) Soluble, exfoliated hexagonal boron nitride nanosheets. J Phys Chem Lett 1:277–283. doi:10.1021/jz9002108|J
Mathesh M, Wang H, Barrow CJ, Yang W (2015) Surface chemistry of two-dimensional layered nanosheets. In: Chen YI (ed) Nanotubes and nanosheets functionalization and applications of boron nitride and other nanomaterials. CRC, Boca Raton, pp 333–366
Choudhary RB, Pande PP (2002) Lubrication potential of boron compounds: an overview. Lubr Sci 14:211–222
Niedenzu K, Dawson JW (1965) Boron-nitrogen compounds, 1st edn. Springer, Berlin-Heidelberg. doi:10.1007/978-3-642-85826-0
Dewar MJS, Lucken EAC, Whitehead MA (1960) The structure of the phosphonitrilic halides. J Chem Soc 2423–2429
Snyder HR, Kuck JA, Johnson JR (1938) Organoboron compounds and the study of reaction mechanisms. Primary aliphatic boronic acids. J Am Chem Soc 60:105–111
López-Castillo A (2012) Prediction of boron-phosphorous nanographene-like material. Int J Quantum Chem 112(19):3152–3157. doi:10.1002/qua.24096
Pupim CF, Morgon NH, Lopez-Castillo A (2015) Spurious phosphorus pyramidalization induced by some DFT functionals. J Braz Chem Soc. doi:10.5935/0103-5053.20150138
Bluhm ME, Bradley MG, Butterick R, Kusari U, Sneddon LG (2006) Amineborane-based chemical hydrogen storage: enhanced ammonia borane dehydrogenation in ionic liquids. J Am Chem Soc 128(24):7748–7749
Weng Q, Wang X, Zhi C, Bando Y, Golberg D (2013) Boron nitride porous microbelts for hydrogen storage. ACS Nano 7(2):1558–1565
Chen X, Gao XP, Zhang H, Zhou Z, Hu WK, Pan GL, Zhu HY, Yan TY, Song DY (2005) Preparation and electrochemical hydrogen storage of boron nitride nanotubes. J Phys Chem B 109(23):11525–11529
Campbell PG, Zakharov LN, Grant DJ, Dixon DA, Liu S-Y (2010) Hydrogen storage by boron-nitrogen heterocycles: a simple route for spent fuel regeneration. J Am Chem Soc 132:3289–3291
Bakus RC, Atwood DA (2006) Boron-nitrogen compounds. Encyclopedia of Inorganic Chemistry:1–16. doi:10.1002/0470862106.ia027
Hosmane N (2011) Boron science: new technologies and applications. CRC, Boca Raton
Uosaki K, Elumalai G, Noguchi H, Masuda T, Lyalin A, Nakayama A, Taketsugu T (2014) Boron nitride nanosheet on gold as an electrocatalyst for oxygen reduction reaction: theoretical suggestion and experimental proof. J Am Chem Soc 136(18):6542–6545. doi:10.1021/ja500393g
Laubengayer AW, MJP C, Porter RF (1961) The condensation of borazine to polycyclic boron-nitrogen frameworks by pyrolytic. J Am Chem Soc 83:1337–1342
Johnson CJ, Zoellner RW (2009) The smallest borazine-fused cyclacenes: novel N–H conformations in cyclo-BN-anthracene and cyclo-BN-tetracene from Hartree-Fock and density functional calculations. J Mol Struct THEOCHEM 893(1–3):9–16. doi:10.1016/j.theochem.2008.09.012
Engelberts JJ, Havenith RW, van Lenthe JH, Jenneskens LW, Fowler PW (2005) The electronic structure of inorganic benzenes: valence bond and ring-current description. Inorg Chem 44(15):5266–5272
Stock A, Pohland E (1926) Borwasserstoffe, VIII. Zur Kenntnis des B2H6 und des B5H11. Eur J Inorg Chem 59(9):2210–2215. doi:10.1002/cber.19260590906
Terrones M, Charlier J-C, Gloter A, Cruz-Silva E, Terrés E, Li YB, Vinu A, Zanolli Z, Dominguez JM, Terrones H, Bando Y, Golberg D (2008) Experimental and theoretical studies suggesting the possibility of metallic boron nitride edges in porous nanourchins. Nano Lett 8(4):1026–1032
Bernard S, Miele P (2014) Polymer-derived boron nitride: a review on the chemistry, shaping and ceramic conversion of borazine derivatives. Materials 7(11):7436–7459. doi:10.3390/ma7117436
Laubengayer AW, Beachley OT (1964) The formation and behavior of polycyclic borazines. Adv Chem Ser 42:281–289. doi:10.1021/ba-1964-0042.ch028
Cragg RH, Nazery M (1986) Organoboron compounds: XXX. Polycyclic borazines. J Organomet Chem 303:329–335
Gonsalves KE, Agarwal R (1988) Polyureidoborazines. Appl Organomet Chem 2:245–249
Pease RS (1952) An x-ray study of boron nitride. Acta Crystallogr 5:356–361
Bauer SH (1938) The structures of the hydrides of boron. IV. B2NH7 and B3N3H6. The structure of dimethylamine. J Am Chem Soc 60:524–530
Schleyer PVR, Maerker C, Dransfeld A, Jiao H, van Eikema Hommes NJR (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318
Schleyer PR, Manoharan M, Wang Z-X, Kiran B, Jiao H, Puchta R, van Eikema Hommes NJR (2001) Dissected nucleus-independent chemical shift analysis of π-aromaticity and antiaromaticity. Org Lett 3(16):2465–2468
Chen Z, Wannere CS, Corminboeuf C, Puchta R, Schleyer PR (2005) Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem Rev 105:3842–3888
Poater J, Duran M, Sola M, Silvi B (2005) Theoretical evaluation of electron delocalization in aromatic molecules by means of atoms in molecules (AIM) and electron localization function (ELF) topological approaches. Chem Rev 105:3911–3947
Kiran B, Phukan AK, Jemmis ED (2001) Is borazine aromatic? Unusual parallel behavior between hydrocarbons and corresponding B-N analogues. Inorg Chem 40:3615–3618
Scott LT, Kirms MA (1981) Azulene thermal rearrangements. 13C-labeling studies of automerization and isomerization to naphthalene. J Am Chem Soc 103:5875–5879
McFarland JD, Welk NAJ, Zoellner RW (1992) MNDO calculations on boron-nitrogen derivatives of nonbenzenoid aromatics: I. The two fully boron-nitrogen-alternating isomers of “inorganic azulene”. Heteroat Chem 3:193–199
McFarland JD, Zoellner RW (1993) Modified neglect of diatomic overlap calculations on boron-nitrogen derivatives of nonbenzenoid aromatics. II. The analysis of the 130 possible nonfullv boron-nitrogen-alternating isomers of pentaazapentaboraazulene. Heteroat Chem 4:145–157
Hättig C (2003) Geometry optimizations with the coupled-cluster model CC2 using the resolution-of-the-identity approximation. J Chem Phys 118(17):7751–7761. doi:10.1063/1.1564061
Hättig C, Köhn A (2002) Transition moments and excited-state first-order properties in the coupled-cluster model CC2 using the resolution-of-the-identity approximation. J Chem Phys 117(15):6939–6951. doi:10.1063/1.1506918
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140(4A):A1133–A1138. doi:10.1103/PhysRev.140.A1133
Ahlrichs R, Bär M, Häser M, Horn H, Kölmel C (1989) Electronic structure calculations on workstation computers: the program system turbomole. Chem Phys Lett 162:165–169
Rappoport D, Furche F (2010) Property-optimized gaussian basis sets for molecular response calculations. J Chem Phys 133(13):134105–134111. doi:10.1063/1.3484283
Becke AD (1993) Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Schreckenbach G, Ziegler T (1995) Calculation of NMR shielding tensors using gauge-including atomic orbitals and modern density functional theory. J Phys Chem 99:606–611
Shen W, Li M, Li Y, Wang S (2007) Theoretical study of borazine and its derivatives. Inorg Chim Acta 360(2):619–624. doi:10.1016/j.ica.2006.08.028
Cassoux P, Kuczkowski RL, Bryan PS, Taylor RC (1975) Microwave spectra of trimethylamine-borane. The boron-nitrogen distance and molecular dipole moment. Inorg Chem 14(1):126–129
Bauer SH (1937) The structure of the hydrides of boron. III. Borine carbonyl and borine trimethylammine. J Am Chem Soc 59:1804–1812
Dinda R, Ciobanu O, Wadepohl H, Hubner O, Acharyya R, Himmel HJ (2007) Synthesis and structural characterization of a stable dimeric boron(II) dication. Angew Chem Int Ed Engl 46(47):9110–9113. doi:10.1002/anie.200703616
Haaland A (1989) Covalent versus dative bonds to main group metals, a useful distinction. Angew Chem Int Ed Engl 28:992–1007
Straub DK (1995) Lewis structures of boron compounds involving multiple bonding. J Chem Educ 72:494–497
Boese R, Maulitz AH, Stellberg P (1994) Solid-state borazine: does it deserve to be entiteled “Inorganic Benzene”? Chem Ber 127:1887–1889
Matsunaga N, Gordon MS (1994) Stabilities and energetics of inorganic benzene isomers: prismanes. J Am Chem Soc 116:11407–11419
Brain PT, Downs AJ, Maccallum P, Rankin DWH, Robertson HE, Forsyth GA (1991) The molecular structures of gaseous tetrakis(dimehylamino)-diboron, B2(NMe2)4, and tetrakis(methoxy)diboron, B2(OMe)4, as determined by electron diffraction. J Chem Soc Dalton Trans 34:1195–1200
Wang Y, Quillian B, Wei P, Wannere CS, Xie Y, King RB, Schaefer HF, Schleyer PR, Robinson GH (2007) A stable neutral diborene containing a B = B double bond. J Am Chem Soc 129:12412–12413
Speight JG (2005) Lange’s handbook of chemistry, 16th edn. McGraw-Hill, New York
Tsuboi M, Overend J (1974) Amino wagging and inversion in hydrazines RR branch of the antisymmetric wagging band of NH2NH2. J Mol Spectrosc 52:256–268
Fallah-Bagher-Shaidaei H, Wannere CS, Corminboeuf C, Puchta R, Schleyer PR (2006) Which NICS aromaticity index for planar π rings is best? Org Lett 8:863–866
Jr Nelson RD, Jr Lide DR, and Maryott AA (1967) Selected Values of electric dipole moments for molecules in the gas phase. NSRDS-NBS10
Möllerstedt H, Piqueras MC, Crespo R, Ottosson H (2004) Fulvenes, fulvalenes, and azulene: are they aromatic chameleons? J Am Chem Soc 126:13938–13939
Gümüş S (2013) A computational study on azaazulenes. Heterocycl Commun 19(5):369–373. doi:10.1515/hc-2013-0100
Acknowledgments
The authors are grateful for the financial support given by CAPES Foundation (Coordenação de Aperfeiçoamento de Pessoal de Nível superior), the São Paulo Research Foundation (FAPESP, Fundação de Amparo a Pesquisa do Estado de São Paulo) Grant 2010/11385-2 and by the CNPq (Brazilian Science Funding Agencies).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
The ESM contains all geometric parameters as well as dipole moment orientation and vertical transition of BN-azulene, NB-azulene and BN-naphthalene molecules. A population analysis of the azulene molecule is also presented for comparison purposes.
Table S1
(DOCX 34 kb)
Table S2
(DOCX 34 kb)
Table S3
(DOCX 38 kb)
Table S4
(DOCX 36 kb)
Table S5
(DOCX 35 kb)
Figure S1
(DOCX 540 kb)
Figure S2
(DOCX 687 kb)
Figure S3
(DOCX 103 kb)
Rights and permissions
About this article
Cite this article
Catão, A.J.L., López-Castillo, A. Stability and molecular properties of the boron-nitrogen alternating analogs of azulene and naphthalene: a computational study. J Mol Model 23, 119 (2017). https://doi.org/10.1007/s00894-017-3279-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3279-y