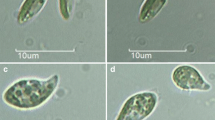
Abstract
The comprehensive study of microorganisms that evolved in the Atacama Desert, the driest and oldest on earth, may help to understand the key role of water for life. In this context, we previously characterized the microenvironment that allows colonization of the underside of quartzes in the Coastal Range of this desert by hypolithic microorganisms (Azua-Bustos et al. Microb Ecol 58:568–581, 2011). Now, we describe the biodiversity composition of these biofilms and the isolation from it of a new cyanobacterial strain. Based on morphologic and phylogenetic analyses, this isolate (AAB1) was classified as a new member of the Gloeocapsopsis genus. Physiological, morphological and molecular responses by isolate AAB1 show that this strain is extremely tolerant to desiccation. Our results also indicate that the isolate biosynthesizes sucrose and trehalose in response to this stressful condition. We identified two candidate genes involved in sucrose synthesis, namely sucrose 6-phosphate synthase and sucrose 6-phosphate phosphatase. Thus, the Gloeocapsopsis isolate AAB1 may represent a suitable model for understanding tolerance to low water availability.







Similar content being viewed by others
References
Alpert P (2005) The limits and frontiers of desiccation-tolerant life. Integr Comp Biol 45:685–695
Alpert P (2006) Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? J Exp Biol 209:1575–1584
Angeloni SV, Potts M (1986) Polysome turnover in immobilized cells of Nostoc commune (cyanobacteria) exposed to water stress. J Bacteriol 168:1036–1039
Ashraf M (2010) Inducing drought tolerance in plants: recent advances. Biotechnol Adv 28:169–183
Avonce N, Mendoza-Vargas A, Morett E, Iturriaga G (2006) Insights on the evolution of trehalose biosynthesis. BMC Evol Biol 6:109
Azua-Bustos A, González-Silva C, Mancilla RA, Salas L, Palma RE, Wynne JJ, McKay CP, Vicuña R (2009) Ancient photosynthetic eukaryote biofilms in an Atacama Desert coastal cave. Microb Ecol 58:485–496
Azua-Bustos A, González-Silva C, Mancilla RA, Salas L, Gómez-Silva B, McKay CP, Vicuña R (2011) Hypolithic cyanobacteria supported mainly by fog in the Coastal Range of the Atacama Desert. Microb Ecol 61:568–581
Azua-Bustos A, González-Silva C, Arenas-Fajardo C, Vicuña R (2012a) Extreme environments as drivers of convergent evolution: the Atacama Desert Coastal Range as a relevant model. Front Microbiol. doi:10.3389/fmicb.2012.00426
Azua-Bustos A, Urrejola C, Vicuña R (2012b) Life at the dry edge: microorganisms of the Atacama Desert. FEBS Lett 586:2939–2945
Bao H, Gu B (2004) Natural perchlorate has a unique oxygen isotope signature. Environ Sci Technol 38:5073–5077
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424
Billi D (2009) Subcellular integrities in Chroococcidiopsis sp. CCMEE 029 survivors after prolonged desiccation revealed by molecular probes and genome stability assays. Extremophiles 13:49–57
Billi D, Grilli Caiola M, Paolozzi L, Ghelardini P (1998) A method for DNA extraction from the desert cyanobacterium Chroococcidiopsis and its application to identification of ftsZ. Appl Environ Microbiol 64:4053–4056
Billi D, Friedmann EI, Helm RF, Potts M (2001) Gene transfer to the desiccation-tolerant cyanobacterium Chroococcidiopsis. J Bacteriol 183:2298–2305
Billi D, Viaggiu E, Cockell CS, Rabbow E, Horneck G, Onofri S (2011) Damage escape and repair in dried Chroococcidiopsis spp. from hot and cold deserts exposed to simulated space and Martian conditions. Astrobiology 11:65–73
Bruno L, Billi D, Albertano P (2005) Optimization of molecular techniques applied to the taxonomy of epilithic Leptolyngbya strains. Arch Hydrobiol Suppl Algol Stud 117:197–207
Buitink J, Leprince O (2004) Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 48:215–228
Bukhov NG, Carpentier R (2004) Effects of water stress on the photosynthetic efficiency of plants. In Advances in: Papageorgiou GC, Govindjee (eds) Photosynthesis and Respiration, Springer, Netherlands, 19: 623–635
Caiola MG, Ocampo-Friedmann R, Friedmann EI (1993) Cytology of long-term desiccation in the desert cyanobacterium Chroococcidiopsis (Chroococcales). Phycologia 32:315–322
Chorus I, Bartram J (1999) Toxic cyanobacteria in water: a guide to public health significance, monitoring and management. E&FN Spon, London
Costerton JW, Stoodley P (2003) Microbial biofilms: protective niches in ancient and modern microbiology. In: Krumbein WE, Paterson DM, Zavarzin GA (eds) Fossil and recent biofilms: a natural history of life on earth. Kluwer, Dordrecht
Coutinho PM, Deleury E, Davies GJ, Henrissat B (2003) An evolving hierarchical family classification for glycosyltransferases. J Mol Biol 328:307–317
Davila AF, Gómez-Silva B, de los Rios A, Ascaso C, Olivares H, McKay CP, Wierzchos J (2008) Facilitation of endolithic microbial survival in the hyperarid core of the Atacama Desert by mineral deliquescence. J Geophys Res 113:G01028
de Los Ríos A, Valea S, Ascaso C, Davila A, Kastovsky J, McKay CP, Gómez-Silva B, Wierzchos J (2010) Comparative analysis of the microbial communities inhabiting halite evaporates of the Atacama Desert. Int Microbiol 13:79–89
Drees KP, Neilson JW, Betancourt JL, Quade J, Henderson DA, Pryor BM, Maier RM (2006) Bacterial community structure in the hyperarid core of the Atacama Desert, Chile. Appl Environ Microbiol 72:7902–7908
Edwards HGM, Moody CD, Villar SE, Wynn-Williams D (2005) Raman spectroscopic detection of key biomarkers of cyanobacteria and lichen symbiosis in extreme Antarctic habitats: evaluation for Mars Lander missions. Icarus 174:560–571
Gorbushina AA (2007) Life on the rocks. Environ Microbiol 9:1613–1631
Hagemann M, Marin K (1999) Salt-induced Sucrose accumulation is mediated by sucrose-phosphate-synthase in cyanobacteria. J Plant Physiol 155:424–430
Harel Y, Ohad I, Kaplan A (2004) Activation of photosynthesis and resistance to photo inhibition in cyanobacteria within biological desert crust. Plant Physiol 136:3070–3079
Hartley A, Chong G, Houston J, Mather A (2005) 150 million years of climatic stability: evidence from the Atacama Desert, northern Chile. J Geol Soc London 162:421–424
Hershkovitz N, Oren A, Cohen Y (1991) Accumulation of trehalose and sucrose in cyanobacteria exposed to matric water stress. Appl Environ Microbiol 57:645–648
Higo A, Katoh H, Ohmori K, Ikeuchi M, Ohmori M (2006) The role of a gene cluster for trehalose metabolism in dehydration tolerance of the filamentous cyanobacterium Anabaena sp. PCC 7120. Microbiology 152:979–987
Higo A, Suzuki T, Ikeuchi M, Ohmori M (2007) Dynamic transcriptional changes in response to rehydration in Anabaena sp. PCC 7120. Microbiology 153:3685–3694
Hirai M, Yamakawa R, Nishio J, Yamaji T, Kashino Y, Koike H, Satoh K (2004) Deactivation of photosynthetic activities is triggered by loss of a small amount of water in a desiccation-tolerant cyanobacterium, Nostoc commune. Plant Cell Physiol 45:872–878
Janse I, Meima M, Kardinaal WE, Zwart G (2003) High-resolution differentiation of cyanobacteria by using rRNA-internal transcribed spacer denaturing gradient gel electrophoresis. Appl Environ Microbiol 69:6634–6643
Klähn S, Hagemann M (2011) Compatible solute biosynthesis in cyanobacteria. Environ Microbiol 13:551–562
Komárek J (1993) Validation of the genera Gloeocapsopsis and Asterocapsa (Cyanoprokaryota) with regard to species from Japan, Mexico and Himalayas. B Natl Sci Museum Ser. B (Botany) 19:19–37
Komárek J (2003) Coccoid and colonial cyanobacteria. In: Wehr JD, Sheath RG (eds) Freshwater algae of North America. Ecology and classification. Academic Press, San Diego
Komárek J, Anagnostidis K (1998) Cyanoprokaryota 1. Teil: Chroococcales. In: Ettl H, Gartner G, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Jena
Lacap DC, Warren-Rhodes KA, McKay CP, Pointing SB (2011) Cyanobacteria and chloroflexi-dominated hypolithic colonization of quartz at the hyper-arid core of the Atacama Desert, Chile. Extremophiles 15:31–38
Lunn JE (2002) Evolution of sucrose synthesis. Plant Physiol 128:1490–1500
Lunn JE, Ashton AR, Hatch MD, Heldt HW (2000) Purification, molecular cloning, and sequence analysis of sucrose-6F-phosphate phosphohydrolase from plants. Proc Natl Acad Sci USA 97:12914–12919
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Dymock D, Wade WG (1998) Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799
Marin K, Huckauf J, Fulda S, Hagemann M (2002) Salt-dependent expression of glucosylglycerol-phosphate synthase, involved in osmolyte synthesis in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 184:2870–2877
Mathlouthi M, Luu DV (1980) Laser-Raman spectra of d-glucose and sucrose in aqueous solution. Carbohyd Res 81:203–212
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
McKay CP, Friedmann EI, Gómez- Silva B, Cáceres-Villanueva L, Andersen DT, Landheim R (2003) Temperature and moisture conditions for life in the extreme arid region of the Atacama Desert: four years of observations including the El Niño of 1997–1998. Astrobiology 3:393–406
Nübel U, Garcia-Pichel F, Muyzer G (1997) PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol 63:3327–3332
Page-Sharp M, Behm CA, Smith GD (1999) Involvement of the compatible solutes trehalose and sucrose in the response to salt stress of a cyanobacterial Scytonema species isolated from desert soils. Biochim Biophys Acta 1472:519–528
Parro V, de Diego-Castilla G, Moreno-Paz M, Blanco Y, Cruz-Gil P, Rodríguez-Manfredi JA, Fernández-Remolar D, Gómez F, Gómez MJ, Rivas LA, Demergasso C, Echeverría A, Urtuvia VN, Ruiz-Bermejo M, García-Villadangos M, Postigo M, Sánchez-Román M, Chong-Díaz G, Gómez-Elvira J (2011) A microbial oasis in the hypersaline Atacama subsurface discovered by a life detector chip: implications for the search for life on Mars. Astrobiology 11:969–996
Potts M (1999) Mechanisms of desiccation tolerance in cyanobacteria. Eur J Phycol 34:319–328
Ramos V, Seabra R, Brito A, Santos A, Santos CL, Lopo M, Moradas-Ferreira P, Vasconcelos VM, Tamagnini P (2010) Characterization of an intertidal cyanobacterium that constitutes a separate clade together with thermophilic strains. Eur J Phycol 45:394–403
Reed RH, Stewart WDP (1983) Physiological responses of Rivularia atra to salinity: osmotic adjustment in hyposaline media. New Phytol 95:595–603
Reed RH, Borowitzka LJ, Mackay MA, Chudek JA, Foster R, Warr SRC, Moore DJ, Stewart WDP (1986) Organic solute accumulation in osmotically stressed cyanobacteria. FEMS Microbiol Rev 39:51–56
Reysenbach AL, Wickham GS, Pace NR (1994) Phylogenetic analysis of the hyperthermophilic pink filament community in octopus spring, Yellowstone National Park. Appl Environ Microbiol 60:2113–2119
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. J Gen Microbiol 111:1–61
Sakamoto T, Yoshida T, Arima H, Hatanaka Y, Takani Y, Tamaru Y (2009) Accumulation of trehalose in response to desiccation and salt stress in the terrestrial cyanobacterium Nostoc commune. Phyc Res 57:66–73
Sakamoto T, Kumihashi K, Kunita S, Masaura T, Inoue-Sakamoto K, Yamaguchi M (2011) The extracellular-matrix-retaining cyanobacterium Nostoc verrucosum accumulates trehalose, but is sensitive to desiccation. FEMS Microbiol Ecol 77:385–394
Salerno GL, Curatti L (2003) Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci 8:63–69
Shirkey B, McMaster NJ, Smith SC, Wright DJ, Rodriguez H, Jaruga P, Birincioglu M, Helm RF, Potts M (2003) Genomic DNA of Nostoc commune (Cyanobacteria) becomes covalently modified during long-term (decades) desiccation but is protected from oxidative damage and degradation. Nucleic Acids Res 31:2995–3005
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Tweddle JC, Dickie JB, Baskin CC, Baskin JM (2003) Ecological aspects of seed desiccation sensitivity. J Ecol 91:294–304
Warr SRC, Reed RH, Stewart WDP (1987) Low-molecular-weight carbohydrate biosynthesis and the distribution of cyanobacteria (blue-green-algae) in marine environments. Br Phycol J 22:175–180
Warren-Rhodes KA, Rhodes KL, Pointing SB, Ewing SA, Lacap DC, Gómez- Silva B, Amundson R, Friedmann EI, McKay CP (2006) Hypolithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama Desert. Microb Ecol 52:389–398
Yoshida T, Sakamoto T (2009) Water-stress induced trehalose accumulation and control of trehalase in the cyanobacterium Nostoc punctiforme IAM M-15. J Gen Appl Microbiol 55:135–145
Zeng XQ, Hou YJ, Yang ZC, Pèli E, Peng Cl, Nie L (2013) Opportunistic growth and desiccation tolerance: the ecological success of poikilohydrous autotrophs. Nova Hedwigia 96:511–524
Acknowledgments
Work on this thesis would not have been possible without the Scholarship for Graduate Studies received from CONICYT Chile by AAB, the financial support from FONDECYT project no 1110597, the Millennium Institute for Fundamental and Applied Biology (MIFAB), Chile and from the AngelicvM Foundation, Chile. We also thank Fabio Rodrigues for his help with Raman analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Electronic supplementary material
Below is the link to the electronic supplementary material.

792_2013_592_MOESM1_ESM.jpg
Supplementary Table 1 Microbial biodiversity of the quartz hypolithic biofilm. Note: “Chroococcales cyanobacterium LEGE 06123” was later classified as Gloeocapsopsis crepidinum LEGE 06123. (JPEG 653 kb)

792_2013_592_MOESM2_ESM.jpg
Supplementary Table 2- GC content (%) of 16S rRNA-ITS-23S rRNA operon sequences. The right column shows the percentage difference in GC content in relation to the sequence of Gloeocapsopsis AAB1. (JPEG 35 kb)


792_2013_592_MOESM4_ESM.jpg
Supplementary Figure 2- DGGE gel of DNA samples obtained by amplification of the ITS gene sequences. Lane 1, hypolithic biofilms; 1.1: Calothrix sp. HA4236-MV1; 1.2: Leptolyngbya sp. Kovacik; 1.3: Nostoc commune NC5 clone 11; 1.4: Gloeocapsopsis AAB1. Lanes 2 – 5, samples obtained from the culture at various times. 2.1, 3.1, 4.1 and 5.1, Gloeocapsopsis AAB1. (JPEG 234 kb)

792_2013_592_MOESM5_ESM.jpg
Supplementary Figure 3- Desiccation chamber. A, chamber assembly. The green color represents the cell sample on top of a microscope slide, whereas the blue marbles are the hygroscopic agent. The arrow points to the internal RH sensor attached to the Petri dish lid. B: temporal profile of % RH inside the chamber with different desiccating agents. No D.A.: no desiccating agent. 1: Silica gel, 1 g. 2: Silica gel, 2 g. 3: Silica gel, 5 g. 4: NaCl, 1 g. 5: NaCl, 2 g. 6: NaCl, 5 g. 7: CaCl, 1 g. 8: CaCl, 2 g. 9: CaCl, 5 g. (JPEG 189 kb)

792_2013_592_MOESM6_ESM.jpg
Supplementary Figure 4 - Absence of DNA and RNA fragmentation of Gloeocapsopsis AAB1 cells desiccated for 13 days at 0.4 aw. Lanes 1 and 3 correspond to the non desiccated control. (JPEG 58 kb)

792_2013_592_MOESM7_ESM.jpg
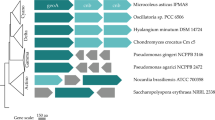
Supplementary Figure 5- Maximum likelihood phylogenetic trees obtained with the aligned sucrose 6-phosphate synthase (A) and sucrose 6-phosphate phosphatase (B) Gloeocapsopsis gene sequences using BOSQUE. The numbers on the nodes represent bootstrap values with 1000 replicates. Gray pentagons besides names denote species from which sequences were obtained from their genomes. (JPEG 221 kb)
Rights and permissions
About this article
Cite this article
Azua-Bustos, A., Zúñiga, J., Arenas-Fajardo, C. et al. Gloeocapsopsis AAB1, an extremely desiccation-tolerant cyanobacterium isolated from the Atacama Desert. Extremophiles 18, 61–74 (2014). https://doi.org/10.1007/s00792-013-0592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0592-y