Abstract
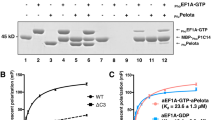
The molecular and functional properties of the elongation factor (EF) G from the psychrophilic Antarctic eubacterium Pseudoalteromonas haloplanktis (Ph) were studied. PhEF-G catalyzed protein synthesis in vitro that was inhibited by fusidic acid, an antibiotic specifically acting on EF-G. The EF interacted with GDP only in the presence of P. haloplanktis ribosome and fusidic acid with an affinity similar to that displayed by Escherichia coli EF-G. The psychrophilic translocase elicited a ribosome-dependent GTPase that was competitively inhibited by GDP, the slowly hydrolyzable GTP analog GppNHp, and the protein synthesis inhibitor ppGDP. The temperature dependence of the activity of PhEF-G reached its maximum at least 26°C beyond the growth temperature of P. haloplanktis (4–20°C). The heat inactivation profile of the ribosome-dependent GTPase of PhEF-G gave a temperature for half inactivation (46°C), significantly lower than that for half denaturation measured by either UV- (57°C) or fluorescence-melting (62°C). This finding was attributed to a different effect of the temperature on the catalytic domain with respect to that elicited on the other domains constituting the EF, thus confirming the differential molecular flexibility present in psychrophilic enzymes. A molecular model, based on the 3D coordinates of a thermophilic EF-G, showed differences only in connecting loops.






Similar content being viewed by others
Abbreviations
- EF:
-
Elongation factor
- Ph:
-
Pseudoalteromonas haloplanktis
- Ec:
-
Escherichia coli
- Tt:
-
Thermus thermophilus
- rPhEF-G:
-
Heterologously expressed PhEF-G
References
Al-Karadaghi S, Aevarsson A, Garber M, Zheltonosova J, Liljas A (1996) The structure of elongation factor G in complex with GDP: conformational flexibility and nucleotide exchange. Structure 15:555–565
Arai N, Arai K, Kaziro Y (1977) Further studies on the interaction of the polypeptide chain elongation factor G with guanine nucleotides. J Biochem (Tokyo) 82:687–694
Arai K, Arai N, Nakamura S, Oshima T, Kaziro Y (1978) Studies on polypeptide-chain-elongation factors from an extreme thermophile, Thermus thermophilus HB8. Eur J Biochem 92:521–531
Arcari P, Masullo M, Arcucci A, Ianniciello G, de Paola B, Bocchini V (1999) A chimeric elongation factor containing the putative guanine nucleotide binding domain of archaeal EF-1 alpha and the M and C domains of eubacterial EF-Tu. Biochemistry 38:12288–12295
Baca OG, Rohrbach MS, Bodley JW (1976) Equilibrium measurements of the interactions of guanine nucleotides with Escherichia coli elongation factor G and the ribosome. Biochemistry 15:4570–4574
Barrick D, Villanueba K, Childs J, Kalil R, Schneider TD, Lawrence CE, Gold L, Stormo GD (1994) Quantitative analysis of ribosome binding sites in E. coli. Nucleic Acids Res 22:1287–1295
Bhargava K, Templeton P, Spremulli LL (2004) Expression and characterization of isoform 1 of human mitochondrial elongation factor G. Protein Expr Purif 37:368–376
Birolo L, Tutino ML, Fontanella B, Gerday C, Mainolfi K, Pascarella S, Sannia G, Vinci F, Marino G (2000) Aspartate aminotransferase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. Cloning, expression, properties and molecular modelling. Eur J Biochem 267:2790–2802
Bocchetta M, Gribaldo S, Sanangelantoni A, Cammarano P (2000) Phylogenetic depth of the bacterial genera Aquifex and Thermotoga inferred from analysis of ribosomal protein, elongation factor, and RNA polymerase subunit sequences. J Mol Evol 50:366–380
Bodley JW, Zieve FJ, Lin L, Zieve ST (1969) Formation of the ribosome-G factor-GDP complex in the presence of fusidic acid. Biochem Biophys Res Commun 37:437–443
Bodley JW, Zieve FJ, Lin L, Zieve ST (1970) Studies on translocation. 3. Conditions necessary for the formation and detection of a stable ribosome-G factor-guanosine diphosphate complex in the presence of fusidic acid. J Biol Chem 245:5656–5661
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Creti R, Ceccarelli E, Bocchetta M, Sanangelantoni AM, Tiboni O, Palm P, Cammarano P (1994) Evolution of translational elongation factor (EF) sequences: reliability of global phylogenies inferred from EF-1 alpha(Tu) and EF-2(G) proteins. Proc Natl Acad Sci USA 91:3255–3259
D’Amico S, Collins T, Marx JC, Feller G, Gerday C (2006) Psychrophilic microorganisms: challenges for life. EMBO Rep 7:385–389
De Vendittis E, Masullo M, Bocchini V (1986) The elongation factor G carries a catalytic site for GTP hydrolysis, which is revealed by using 2-propanol in the absence of ribosomes. J Biol Chem 261:4445–4450
De Vendittis E, Adinolfi BS, Amatruda MR, Raimo G, Masullo M, Bocchini V (1999) The A26G replacement in the consensus sequence A-X-X-X-X-G-K-[T,S] of the guanine nucleotide binding site activates the intrinsic GTPase of the elongation factor 2 from the archaeon Sulfolobus solfataricus. Eur J Biochem 262:600–605
De Vendittis E, de Paola B, Gogliettino MA, Adinolfi BS, Fiengo A, Duvold T, Bocchini V (2002) Fusidic and helvolic acid inhibition of elongation factor 2 from the archaeon Sulfolobus solfataricus. Biochemistry 41:14879–14884
Dever TE, Glynias MJ, Merrick WC (1987) GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci USA 84:1814–1818
Feller G, Gerday C (1997) Psychrophilic enzymes: molecular basis of cold adaptation. Cell Mol Life Sci 53:830–841
Feller G, Narinx E, Arpigny JL, Aittaleb M, Baise E, Gerday C (1996) Enzymes from psychrophilic organisms. FEMS Microbiol Rev 18:189–202
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Hansson S, Singh R, Gudkov AT, Liljas A, Logan DT (2005) Structural insights into fusidic acid resistance and sensitivity in EF-G. J Mol Biol 348:939–949
Herbert RA, Sharp RS (1992) Molecular biology and biotechnology of extremophiles. Blakie Press, New York, USA
Hoyoux A, Blaise V, Collins T, D’Amico S, Gratia E, Huston AL, Marx JC, Sonan G, Zeng Y, Feller G, Gerday C (2004) Extreme catalysts from low-temperature environments. J Biosci Bioeng 98:317–330
Jaenicke R, Zavodszky P (1990) Proteins under extreme physical conditions. FEBS Lett 268:344–349
Johanson U, Hughes D (1994) Fusidic acid-resistant mutants define three regions in elongation factor G of Salmonella typhimurium. Gene 143:55–59
Johanson U, Aevarsson A, Liljas A, Hughes D (1996) The dynamic structure of EF-G studied by fusidic acid resistance and internal revertants. J Mol Biol 258:420–432
Kaziro Y (1978) The role of guanosine 5′-triphosphate in polypeptide chain elongation. Biochim Biophys Acta 505:95–127
Klink F (1985) Elongation factors. In: Woese CR, Wolfe R (eds) The bacteria, vol 8. Academic, London, pp 379–410
Lucas-Lenard J (1971) Protein biosynthesis. Annu Rev Biochem 40:409–448
Martemyanov KA, Liljas A, Yarunin AS, Gudkov AT (2001) Mutations in the G-domain of elongation factor G from Thermus themophilus affect both its interaction with GTP and fusidic acid. J Biol Chem 276:28774–28778
Masullo M, Parlato G, De Vendittis E, Bocchini V (1989) Effect of propan-2-ol on enzymic and structural properties of elongation factor G. Biochem J 261:725–731
Masullo M, De Vendittis E, Bocchini V (1994) Archaebacterial elongation factor 1 alpha carries the catalytic site for GTP hydrolysis. J Biol Chem 269:20376–20379
Masullo M, Arcari P, de Paola B, Parmeggiani A, Bocchini V (2000) Psychrophilic elongation factor Tu from the Antarctic Pseudoalteromonas haloplanktis sp. TAC II 25. Biochemical characterization and cloning of the encoding gene. Biochemistry 39:15531–15539
Medigue C, Krin E, Pascal G, Barbe V, Bernsel A, Bertin PN, Cheung F, Cruveiller S, D’Amico S, Duilio A, Fang G, Feller G, Ho C, Mangenot S, Marino G, Nilsson J, Parrilli E, Rocha EP, Rouy Z, Sekowska A, Tutino ML, Vallenet D, von Heijne G, Danchin A (2005) Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res 15:1325–1335
Miller DL, Weissbach H (1977) In: Weissbach H, Pestka S (eds) Molecular mechanisms of protein biosynthesis. Academic, New York, pp 323–373
Nagaev I, Bjorkman J, Andersson DI, Hughes D (2001) Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol Microbiol 40:433–439
Parmeggiani A, Sander G (1981) Properties and regulation of the GTPase activities of elongation factors Tu and G, and of initiation factor 2. Mol Cell Biochem 35:129–158
Peitsch MC (1996) ProMod and Swiss-Model: internet-based tools for automated comparative protein modeling. Biochem Soc Trans 24:274–279
Peske F, Savelsbergh A, Katunin VI, Rodnina MV, Wintermeyer W (2004) Conformational changes of the small ribosomal subunit during elongation factor G-dependent tRNA-mRNA translocation. J Mol Biol 343:1183–1194
Post LE, Nomura N (1980) DNA sequences from the str operon of Escherichia coli. J Biol Chem 255:4660–4666
Pribnow D (1975) Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol 99:419–433
Raimo G, Masullo M, Parente A, Dello Russo A, Bocchini V (1992) Molecular, functional and structural properties of an archaebacterial elongation factor 2. Biochim Biophys Acta 1132:127–132
Raimo G, Masullo M, Bocchini V (1995) Studies on the polypeptide elongation factor 2 from Sulfolobus solfataricus. Interaction with guanosine nucleotides and GTPase activity stimulated by ribosomes. J Biol Chem 270:21082–21085
Raimo G, Masullo M, Scarano G, Bocchini V (1996) The site for GTP hydrolysis on the archaeal elongation factor 2 is unmasked by aliphatic alcohols. Biochimie 78:832–837
Raimo G, Lombardo B, Masullo M, Lamberti A, Longo O, Arcari P (2005) Elongation factor Ts from the Antarctic eubacterium Pseudoalteromonas haloplanktis TAC125: biochemical characterization and cloning of the encoding gene. Biochemistry 43:14759–14766
Sander G, Marsh RC, Voigt J, Parmeggiani A (1975) A comparative study of the 50S ribosomal subunit and several 50S subparticles in EF-T-and EF-G-dependent activities. Biochemistry 14:1805–1814
Shine J, Dalgarno L (1974) The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA 71:1342–1346
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 26:403–433
Stark H, Rodnina MV, Wieden HJ, van Heel M, Wintermeyer W (2000) Large-scale movement of elongation factor G and extensive conformational change of the ribosome during translocation. Cell 100:301–309
Tanford C (1962) Contribution of hydrophobic interactions to the stability of the globular conformation of proteins. J Am Chem Soc 84:4240–4247
Thomas T, Cavicchioli R (2000) Effect of temperature on stability and activity of elongation factor 2 proteins from Antarctic and thermophilic methanogens. J Bacteriol 182:1328–1332
Tosco A, Birolo L, Madonna S, Lolli G, Sannia G, Marino G (2003) GroEL from the psychrophilic bacterium Pseudoalteromonas haloplanktis TAC 125: molecular characterization and gene cloning. Extremophiles 7:17–28
Willie GR, Richman N, Godtfredsen WO, Bodley JW (1975) Some characteristics of and structural requirements for the interaction of 24,25-dihydrofusidic acid with ribosome—elongation factor G complexes. Biochemistry 14:1713–1718
Zuber H (1988) Temperature and adaptation of lactate dehydrogenase. Structural, functional and genetic aspects. Biophys Chem 29:171–179
Acknowledgments
This work was supported by PRIN 2005, MIUR, Rome (Italy). The P. haloplanktis strain was kindly supplied by Prof. Charles Gerday, University of Liege (Belgium).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by G. Antranikian.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruggiero, I., Raimo, G., Palma, M. et al. Molecular and functional properties of the psychrophilic elongation factor G from the Antarctic Eubacterium Pseudoalteromonas haloplanktis TAC 125. Extremophiles 11, 699–709 (2007). https://doi.org/10.1007/s00792-007-0088-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-007-0088-8