Abstract
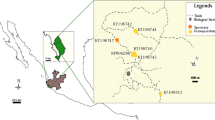
The leafy liverwort Lophozia excisa, which is colonised by basidiomycete fungi in other biomes and which evidence suggests may be colonised by mycorrhizal fungi in Antarctica, was sampled from Léonie Island in the southern maritime Antarctic (67°36′ S, 68°21′ W). Microscopic examination of plants indicated that fungal hyphae colonised 78% of the rhizoids of the liverwort, apparently by entering the tips of rhizoids prior to growing into their bases, where they formed hyphal coils. Extensive colonisation of stem medullary cells by hyphae was also observed. DNA was extracted from surface-sterilised liverwort tissues and sequenced following nested PCR, using the primer set ITS1F/TW14, followed by a second round of amplification using the ITSSeb3/TW13 primer set. Neighbour-joining analyses showed that the sequences obtained nested in Sebacinales clade B as a 100% supported sister group to Sebacinales sequences from the leafy liverworts Lophozia sudetica, L. incisa and Calypogeia muelleriana sampled from Europe. Direct PCR using the fungal specific primer set ITS1F/ITS4 similarly identified fungi belonging to Sebacinales clade B as the principal colonists of L. excisa tissues. These observations indicate the presence of a second mycothallus in Antarctica and support the previous suggestion that the Sebacinales has a wide geographical distribution.



Similar content being viewed by others
References
Allen SE (1989) Chemical analysis of ecological materials. Blackwell, Oxford, p 368
Allen TR, Millar T, Berch SM, Berbee ML (2003) Culturing and direct DNA extraction find different fungi from the same ericoid mycorrhizal roots. New Phytol 160:255–272
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
Barazani O, Benderoth M, Groten K, Kuhlemeier C, Baldwin IT (2005) Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 146:234–243
Bednarek-Ochyra H, Váňa J, Ochyra JVR, Smith RIL (2000) The liverwort flora of Antarctica. Institute of Botany, Polish Academy of Sciences, Cracow, p 236
Boullard B (1988) Observations on the coevolution of fungi with hepatics. In: Pyrozynski KA (ed) Coevolution of fungi with plants and animals. Academic, London, pp 107–124
Chambers SM, Williams PG, Seppelt RD, Cairney JWG (1999) Molecular identification of Hymenoscyphus sp. from rhizoids of the leafy liverwort Cephaloziella exiliflora in Australia and Antarctica. Mycol Res 103:286–288
Cullings KW (1994) Molecular phylogeny of the Monotropoideae (Ericaceae) with a note on the placement of the Pyroloideae. J Evol Biol 7:501–516
Davey ML, Currah RS (2007) A new species of Cladophialophora (hyphomycetes) from boreal and montane bryophytes. Mycol Res 111:106–116
Deshmukh S, Hückelhoven R, Schäfer P, Imani J, Sharma M, Weiß M, Waller F, Kogel K-H (2006) The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc Nat Acad Sci USA 103:18450–18457
Duckett JG, Read DJ (1991) The use of the fluorescent dye, 3, 3′dihexyloxacarbocyanine iodide, for selective staining of ascomycete fungi associated with liverwort rhizoids and ericoid mycorrhizal roots. New Phytol 118:259–272
Duckett JG, Russell J, Ligrone R (2006) Basidiomycetous endophytes in jungermannialean (leafy) liverworts have novel cytology and species-specific host ranges: a cytological and experimental study. Can J Bot 84:1075–1093
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for Basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Huiskes AHL, Lud D, Moerdijk-Poortvliet TCW (2001) Field research on the effects of UV-B filters on terrestrial Antarctic vegetation. Plant Ecol 154:77–86
Kottke I, Beiter A, Weiß M, Haug I, Oberwinkler F, Nebel M (2003) Heterobasidiomycetes form symbiotic associations with hepatics: Jungermanniales have sebacinoid mycobionts while Aneura pinguis (Metzgeriales) is associated with a Tulasnella species. Mycol Res 107:957–968
Montiel P, Smith A, Keiller D (1999) Photosynthetic responses of selected Antarctic plants to solar radiation in the southern maritime Antarctic. Polar Res 18:229–235
Newsham KK (2009) The biology and ecology of the liverwort Cephaloziella varians in Antarctica. Antarct Sci 22. doi:10.1017/S0954102009990630
Pearson WR (1990) Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol 183:63–98
Pressel S, Ligrone R, Duckett JG (2008) The ascomycete Rhizoscyphus ericae elicits a range of host responses in the rhizoids of leafy liverworts: an experimental and cytological analysis. Fieldiana: Botany 47:59–72
Read DJ, Duckett JG, Francis R, Ligrone R, Russell A (2000) Symbiotic fungal associations in ‘lower’ land plants. Phil Trans R Soc Lond B 355:815–831
Selosse M-A, Setaro S, Glatard F, Richard F, Urcelay C, Weiß M (2007) Sebacinales are common mycorrhizal associates of Ericaceae. New Phytol 174:864–878
Setaro S, Weiß M, Oberwinkler F, Kottke I (2006) Sebacinales form ectendomycorrhizas with Cavendishia nobilis, a member of the Andean clade of Ericaceae, in the mountains rain forest of southern Ecuador. New Phytol 169:355–365
Smith RIL (1984) Terrestrial plant biology of the sub-Antarctic and Antarctic. In: Laws RM (ed) Antarctic ecology vol 1. Academic, London, pp 61–162
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Upson R, Read DJ, Newsham KK (2007) Widespread association between the ericoid mycorrhizal fungus Rhizoscyphus ericae and a leafy liverwort in the maritime and sub-Antarctic. New Phytol 176:460–471
Warcup JH (1988) Mycorrhizal associations of isolates of Sebacina vermifera. New Phytol 110:227–231
Weiß M, Selosse M-A, Rexer K-H, Urban U, Oberwinkler F (2004) Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycol Res 108:1003–1010
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand H, Sninsky JS, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Acknowledgements
Funding was supplied by the Natural Environment Research Council through the British Antarctic Survey’s Long-Term Monitoring and Survey programme. Ryszard Ochyra identified the samples of L. excisa, and Katarzyna Turnau provided advice on staining procedures. The staff of the BAS Logistics Group and Rothera Research base, particularly Jim Elliot and James Wake, assisted with transport to and from Léonie Island. Peter Fretwell drew Fig. 1 and Sandra McInnes and Jamie Oliver helped with the preparation of Fig. 2. Two anonymous referees supplied helpful comments. All are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newsham, K.K., Bridge, P.D. Sebacinales are associates of the leafy liverwort Lophozia excisa in the southern maritime Antarctic. Mycorrhiza 20, 307–313 (2010). https://doi.org/10.1007/s00572-009-0283-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-009-0283-9