Abstract
Key message
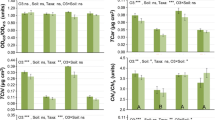
Elevated CO 2 enhances the photosynthesis and growth of hybrid larch F 1 seedlings. However, elevated CO 2 -induced change of tree shape may have risk to the other environmental stresses.
Abstract
The hybrid larch F1 (Larix gmelinii var. japonica × L. kaempferi) is one of the most promising species for timber production as well as absorption of atmospheric CO2. To assess the ability of this species in the future high CO2 environment, we investigated the growth and photosynthetic response of hybrid larch F1 seedlings to elevated CO2 concentration. Three-year-old seedlings of hybrid larch F1 were grown on fertile brown forest soil or infertile volcanic ash soil, and exposed to 500 μmol mol−1 CO2 in a free-air CO2 enrichment system located in northern Japan for two growing seasons. Regardless of soil type, the exposure to elevated CO2 did not affect photosynthetic traits in the first and second growing seasons; a higher net photosynthetic rate was maintained under elevated CO2. Growth of the seedlings under elevated CO2 was greater than that under ambient CO2. We found that elevated CO2 induced a change in the shape of seedlings: small roots, slender-shaped stems and long-shoots. These results suggest that elevated CO2 stimulates the growth of hybrid larch F1, although the change in tree shape may increase the risk of other stresses, such as strong winds, heavy snow, and nutrient deficiency.



Similar content being viewed by others
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell Environ 30:258–270
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259
Brasier C, Webber J (2010) Sudden larch death. 466:824–825
Calfapietra C, Gielen B, Galema ANJ, Lukac M, De Angelis P, Moscatelli MC, Ceulemans R, Scarascia-Mugnozza G (2003) Free-air CO2 enrichment (FACE) enhances biomass production in a short-rotation poplar plantation. Tree Physiol 23:805–814
Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, Held I, Jones R, Kolli RK, Kwon W-T, Laprise R, Magaña Rueda V, Mearns L, Menéndez CG, Räisänen J, Rinke A, Sarr A, Whetton P (2007) Regional climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 847–940
Crous K, Walters MB, Ellsworth DS (2008) Elevated CO2 concentration affects leaf photosynthesis–nitrogen relationships in Pinus taeda over nine years in FACE. Tree Physiol 28:607–614
Eguchi N, Fukatsu E, Funada R, Tobita H, Kitao M, Maruyama Y, Koike T (2004) Changes in morphology, anatomy, and photosynthetic capacity of needles of Japanese larch (Larix kaempferi) seedlings grown in high CO2 concentration. Photosynthetica 42:173–178
Eguchi N, Funada R, Ueda T, Takagi K, Hiura T, Sasa K, Koike T (2005) Soil moisture condition and growth of deciduous tree seedlings native to northern Japan raised under elevated CO2 with a FACE system. Phyton 45:133–138
Eguchi N, Karatsu K, Ueda T, Funada R, Takagi K, Hiura T, Sasa K, Koike T (2008) Photosynthetic responses of birch and alder saplings grown in a free air CO2 enrichment system in northern Japan. Trees 22:437–447
Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD (2004) Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Global Change Biol 10:2121–2138
Ellsworth DS, Thomas R, Crous KY, Palmroth S, Ward E, Maier C, DeLucia E, Oren R (2012) Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: a synthesis from Duke FACE. Glob Chang Biol 18:223–242
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Gower ST, Richards JH (1990) Larches: deciduous conifers in an evergreen world. Bioscience 40:818–826
Handa IT, Körner C, Hättenschwiler S (2005) A test of the treeline carbon limitation hypothesis by in situ CO2 enrichment and defoliation. Ecology 86:1288–1300
Handa IT, Körner C, Hättenschwiler S (2006) Conifer stem growth at the altitudinal treeline in response to four years of CO2 enrichment. Glob Change Biol 12:2417–2430
Handa IT, Hagedorn F, Hattenschwiler S (2008) No stimulation in root production in response to 4 years of in situ CO2 enrichment at the Swiss treeline. Funct Ecol 22:348–358
Kaji K (1983) Wind and snow resistances of hybrid larch. Koushunai Kiho 56:1–5
Karnosky DF, Pregitzer KS, Zak DR, Kubiske ME, Hendrey GR, Weinstein D, Nosal M, Percy KE (2005) Scaling ozone responses of forest trees to the ecosystem level in a changing climate. Plant Cell Environ 28:965–981
Kato Y (1983) Generation mechanism of volcanic ash soil. In: Takai Y (ed) Volcanic ash soil. Hakuyusha, Tokyo, pp 5–30 (In Japanese)
Kayama M, Makoto K, Nomura M, Satoh F, Koike T (2009) Dynamics of elements in larch seedlings (Larix kaempferi) regenerated on serpentine soil in northern Japan. Landscape Ecol Eng 5:125–135
King JS, Kubiske MF, Pregitzer KS, Hendrey GR, McDonald EP, Giardina CP, Quinn VS, Karnosky DF (2005) Tropospheric O3 compromises net primary production in young stands of trembling aspen, paper birch and sugar maple in response to elevated atmospheric CO2. New Phytol 168:623–635
Kita K, Fujimoto T, Uchiyama K, Kuromaru M, Akutsu H (2009) Estimated amount of carbon accumulation of hybrid larch in three 31-year-old progeny test plantations. J Wood Sci 55:425–434
Kitaoka S, Mori S, Matsuura Y, Abaimov AP, Sugishita Y, Satoh F, Sasa K, Koike T (2000) Comparison between the photosynthetic characteristics of larch species grown in northern Japan and central Siberia. Proc Joint Sib Permafrost Stud 8:49–54
Lambers H, Chapin FS III, Pons TL (2008) Plant physiological ecology, 2nd edn. Springer, New York
Liberloo M, Lukac M, Calfapietra C, Hoosbeek MR, Gielen B, Miglietta F, Scarascia-Mugnozza GE, Ceulemans R (2009) Coppicing shifts CO2 stimulation of poplar productivity to above-ground pools: a synthesis of leaf to stand level results from the POP/EUROFACE experiment. New Phytol 182:331–346
Lilleskov EA, Fahey TJ, Horton TR, Lovett GM (2002) Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 83:104–115
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54:2393–2401
Makita A (2004) Characteristics of life history for Sasa. In: Koike T (ed) Tree physiological ecology. Asakura Publishing Co., Ltd., Tokyo, pp 199–210 (in Japanese)
Matsuda K, Shibuya M, Koike T (2002) Maintenance and rehabilitation of the mixed conifer-broadleaf forests in Hokkaido, northern Japan. Eurasian J For Res 5:119–130
Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A, Raper SCB, Watterson IG, Weaver AJ, Zhao Z-C (2007) Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge and New York, pp 747–846
Mitchell SJ (2000) Stem growth responses in Douglas-fir and Sitka spruce following thinning: implications for assessing wind-firmness. For Ecol Manag 135:105–114
Miyaki Y (1990) Superior characters of the F1 hybrid Larix gmelinii × L. kaempferi and the growth gain in full-sib family selection. For Tree Breed Hokkaido 33:7–12 (in Japanese)
Monastersky R (2013) Global carbon dioxide levels near worrisome milestone. Nature 497:13–14
Norby RJ, Zak DR (2011) Ecological lessons from free-air CO2. Enrichment (FACE) experiments. Annu Rev Ecol Evol Syst 42:181–203
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. PNAS 107:19368–19373
Nowak RS, Ellsworth DS, Smith SD (2004) Functional responses of plants to elevated atmospheric CO2—do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol 162:253–280
Ryu K, Watanabe M, Shibata H, Takagi K, Nomura M, Koike T (2009) Ecophysiological responses of the larch species in northern Japan to environmental changes as a basis for afforestation. Landsc Ecol Eng 5:99–106
Shibuya M, Urata T, Torita H, Iijima H (2011) Windthrow damage and tree shape in coniferous plantations in central Hokkaido, northern Japan. Jpn J For Environ 53:53–59 (in Japanese)
Sterck F (2005) Woody tree architecture. In: Turnbull CGN (ed) Plant architecture and its manipulation. Blackwell Publishing Ltd, Oxford, pp 209–237
Tissue DT, Lewis JD (2010) Photosynthetic responses of cottonwood seedlings grown in glacial through future atmospheric [CO2] vary with phosphorus supply. Tree Physiol 30:1361–1372
Tissue DT, Oechel WC (1987) Response of Eriophorum vaginatum to elevated CO2 and temperature in the Alaskan tussock tundra. Ecology 68:401–410
Tissue DT, Thomas RB, Strain BR (1993) Long-term effects of elevated CO2 and nutrients on photosynthesis and rubisco in loblolly pine seedlings. Plant Cell Environ 16:859–865
Tjoelker MG, Oleksyn J, Reich PB (1998a) Seedlings of five boreal tree species differ in acclimation of net photosynthesis to elevated CO2 and temperature. Tree Physiol 18:715–726
Tjoelker MG, Oleksyn J, Reich PB (1998b) Temperature and ontogeny mediate growth response to elevated CO2 in seedlings of five boreal tree species. New Phytol 140:197–210
Watanabe Y, Satomura T, Sasa K, Funada R, Koike T (2010) Differential anatomical responses to elevated CO2 in saplings of four hard wood species. Plant Cell Environ 33:1101–1111
Watanabe M, Watanabe Y, Kitaoka S, Utsugi H, Kita K, Koike T (2011) Growth and photosynthetic traits of hybrid larch F1 under elevated CO2 concentration with low nutrient availability. Tree Physiol 31:965–975
Watanabe M, Ryu K, Kita K, Takagi K, Koike T (2012) Effects of nitrogen load on the growth and photosynthesis of hybrid larch F1 (Larix gmelinii var. japonica × L. kaempferi) grown on serpentine soil. Environ Exp Bot 83:73–81
Yamanobe T (2006) Selection of new forestry species of Cedar for making multilayer forests. Growth of Cedar seedlings under canopy shade after the exposure to sunlight. Tech Rep Forest Tree Breed 28:7 (in Japanese)
Yazaki K, Funada R, Mori S, Maruyama Y, Abaimov AP, Kayama M, Koike T (2001) Growth and annual ring structure of Larix sibirica grown at different carbon dioxide concentrations and nutrient supply rates. Tree Physiol 21:1223–1229
Yazaki K, Ishida S, Kawagishi T, Fukatsu E, Maruyama Y, Kitao M, Tobita H, Koike T, Funada R (2004) Effects of elevated CO2 concentration on growth, annual ring structure and photosynthesis in Larix kaempferi seedlings. Tree Physiol 24:951–959
Acknowledgments
The authors are greatly indebted to Mr. M. Imori (Silviculture and Forest Ecological Studies, Hokkaido University) for his technical support. This study was supported partly by Grant-in-Aid from the Japan Society for the Promotion of Science through the programs of a Grant-in-Aid for Young Scientists (B) (24710027, to M. Watanabe), and a Grant-in-Aid for Scientific Research on Innovative Areas (21114008, to T. Koike), and by Agriculture, Forestry and Fisheries Research Council through its project study of Development of Mitigation and Adaptation Techniques to Global Warming in the Sectors of Agriculture, Forestry, and Fisheries (to K. Kita).
Conflict of interest
The authors declare that they have no conflict of interest.
Author contribution statement
MW promoted this study, determined photosynthetic traits, analyzed all the data, and wrote the entire manuscript. MQ and EN worked for determination of biomass, and discussed data interpretation. KK prepared seedlings of hybrid larch F1 for this study, and discussed data interpretation. KT and FS managed the experimental system for CO2 fumigation. TK is representative person of this project, and proposed the concept of this study, and discussed data interpretation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Buckeridge.
Rights and permissions
About this article
Cite this article
Watanabe, M., Mao, Q., Novriyanti, E. et al. Elevated CO2 enhances the growth of hybrid larch F1 (Larix gmelinii var. japonica × L. kaempferi) seedlings and changes its biomass allocation. Trees 27, 1647–1655 (2013). https://doi.org/10.1007/s00468-013-0912-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-013-0912-y