Abstract
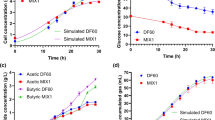
Reducing power such as NADH is an essential factor for acetone/butanol/ethanol (ABE) fermentation using Clostridium spp. The objective of this study was to increase available NADH in Clostridium beijerinckii IB4 by a microbial electrolysis cell (MEC) with an electron carrier to enhance butanol production. First of all, a MEC was performed without electron carrier to study the function of cathodic potential applying. Then, various electron carriers were tested, and neutral red (NR)-amended cultures showed an increase of butanol concentration. Optimal NR concentration (0.1 mM) was used to add in a MEC. Electricity stimulated the cell growth obviously and dramatically diminished the fermentation time from 40 to 28 h. NR and electrically reduced NR improved the final butanol concentration and inhibited the acetone generation. In the MEC with NR, the butanol concentration, yield, proportion and productivity were increased by 12.2, 17.4, 7.2 and 60.3 %, respectively. To further understand the mechanisms of NR, cathodic potential applying and electrically reduced NR, NADH and NAD+ levels, ATP levels and hydrogen production were determined. NR and electrically reduced NR also improved ATP levels and the ratio of NADH/NAD+, whereas they decreased hydrogen production. Thus, the MEC is an efficient method for enhancing the butanol production.







Similar content being viewed by others
References
Wu H, Chen XP, Liu GP, Jiang M, Guo T, Jin WQ, Wei P, Zhu DW (2012) Acetone–butanol–ethanol (ABE) fermentation using Clostridium acetobutylicum XY16 and in situ recovery by PDMS/ceramic composite membrane. Bioprocess Biosyst Eng 35:1057–1065
Jiang M, Chen JN, He AY, Wu H, Kong XP, Liu JL, Yin CY, Chen WF, Chen P (2014) Enhanced acetone/butanol/ethanol production by Clostridium beijerinckii IB4 using pH control strategy. Process Biochem 49:1238–1244
Dürre P (2007) Biobutanol: an attractive biofuel. Biotech J 2:1525–1534
Jiang Y, Xu CM, Dong F, Yang YL, Jiang WH, Yang S (2009) Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 11:284–291
Tracy BP (2012) Improving butanol fermentation to enter the advanced biofuel market. MBio 3:e00518–12
Li TG, Yan Y, He JZ (2014) Reducing cofactors contribute to the increase of butanol production by a wild-type Clostridium sp. strain BOH3. Bioresour Technol 155:220–228
Ezeji TC, Qureshi N, Blaschek HP (2007) Bioproduction of butanol from biomass: from genes to bioreactors. Curr Opin Biotechnol 18:220–227
Li L, Ai HX, Zhang SX, Li S, Liang ZX, Wu ZQ, Yang ST, Wang JF (2013) Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour Technol 143:397–404
Zheng J, Tashiro Y, Wang QH, Sonomoto K (2014) Recent advances to improve fermentative butanol production: genetic engineering and fermentation technology. J Biosci Bioeng 119:1–9
Tran HTM, Cheirsilp B, Hodgson B, Umsakul K (2010) Potential use of Bacillus subtilis in a co-culture with Clostridium butylicum for acetone-butanol-ethanol production from cassava starch. Biochem Eng J 48:260–267
Abd-Alla MH, Elsadek El-Enany AW (2012) Production of acetone-butanol-ethanol from spoilage date palm (Phoenix dactylifera L.) fruits by mixed culture of Clostridium acetobutylicum and Bacillus subtilis. Biomass Bioenergy 42:172–178
Nakayama SI, Kiyoshi K, Kadokura T, Nakazato A (2011) Butanol production from crystalline cellulose by cocultured Clostridium thermocellum and Clostridium saccharoperbutylacetonicum N1-4. Appl Environ Microbiol 77:6470–6475
Lee JY, Jang YS, Lee J, Papoutsakis ET, Lee SY (2009) Metabolic engineering of Clostridium acetobutylicum M5 for highly selective butanol production. Biotech J 4:1432–1440
Jang YS, Lee JY, Lee J, Park JH, Im JA, Eom MH, Lee J, Lee SH, Song H, Cho JH, Seung DY, Lee SY (2012) Enhanced butanol production obtained by reinforcing the direct butanol-forming route in Clostridium acetobutylicum. MBio 3:e00314–12
Kong XP, He AY, Zhao J, Wu H, Jiang M (2015) Efficient acetone–butanol–ethanol production (ABE) by Clostridium acetobutylicum XY16 immobilized on chemically modified sugarcane bagasse. Bioprocess Biosyst Eng. doi:10.1007/s00449-015-1377-8
Jang YS, Malaviya A, Lee SY (2013) Acetone-butanol-ethanol production with high productivity using Clostridium acetobutylicum BKM19. Biotechnol Bioeng 110:1646–1653
Bankar SB, Survase SA, Singhal RS, Granstrom T (2012) Continuous two stage acetone-butanol-ethanol fermentation with integrated solvent removal using Clostridium acetobutylicum B5. Bioresour Technol 106:110–116
Martin JR, Petitdemange H, Ballongue J, Gay R (1983) Effects of acetic and butyric acids on solvents production by Clostridium acetobutylicum. Biotechnol Lett 5:89–94
Al-Shorgani NKN, Ali E, Kalil MS, Yusoff WMW (2011) Bioconversion of butyric acid to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) in a limited nutrient medium. Bioeng Res 5:287–293
Lutke-Eversloh T, Bahl H (2011) Metabolic engineering of Clostridium acetobutylicum: recent advances to improve butanol production. Curr Opin Biotechnol 22:634–647
Girbal L, Vasconcelos I, Saint-Amans S, Soucaille P (1995) How neutral red modified carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH. FEMS Microbiol Rev 16:151–162
Rao G, Mutharasan R (1987) Altered Electron Flow in Continuous Cultures of Clostridium acetobutylicum Induced by Viologen Dyes. Appl Environ Microbiol 53:1232–1235
Ballongue J, Amine J, Petitdemange H, Gay R (1986) Enhancement of solvents production by clostridium acetobutylicum cultivated on a reducing compounds depletive medium. Biomass 10:121–129
Rao G, Mutharasan R (1986) Alcohol production by Clostridium acetobutylicum induced by methyl viologen. Biotechnol Lett 8:893–896
Lovley DR (2011) Powering microbes with electricity: direct electron transfer from electrodes to microbes. Environ Microbiol Rep 3:27–35
Steinbusch KJJ, Hamelers HVM, Schaap JD, Kampman C, Buisman CJN (2010) Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures. Environ Sci Technol 44:513–517
Rabaey K, Rozendal RA (2010) Microbial electrosynthesis-revisiting the electrical route for microbial production. Nat Rev Microbiol 8:706–716
Cournet A, Délia ML, Bergel A, Roques C, Bergé M (2010) Electrochemical reduction of oxygen catalyzed by a wide range of bacteria including Gram-positive. Electrochem Commun 12:505–508
Fultz ML, Durst RA (1982) Mediator compounds for the electrochemical study of biological redox systems: a compilation. Anal Chim Acta 140:1–18
Gründig B, Wittstock G, Rü del U, Strehlitz B (1995) Mediator-modified electrodes for electrocatalytic oxidation of NADH. J Electroanal Chem 395:143–157
Jin XQ, Zhou H, Wu X, Zhang G, He BF (2008) A rapid screening method of producing strain in acetone-butanol fermentation. Chin J Process Eng 6:1185–1189
Qureshi N, Blaschek HP (2001) Recent advances in ABE fermentation: hyper-butanol producing Clostridium beijerinckii BA101. J Ind Microbiol Biotechnol 27:287–291
Xie XH, Li EL, Tang ZK (2010) Sudden emergence of redox active escherichia coli phenotype: cyclic voltammetric evidence of the overlapping pathways. Int J Electrochem Sci 5:1070–1081
Li J, Jiang M, Chen KQ, Ye Q, Shang LA, Wei P, Yin HJ, Chang HN (2010) Effect of redox potential regulation on succinic acid production by Actinobacillus succinogenes. Bioprocess Biosyst Eng 33:911–920
Park DH, Laivenieks M, Guettler MV, Jain MK, Zeikus JG (1999) Growth and metabolite production neutral red as the sole electron donor for microbial utilization of electrically reduced. Appl Environ Microbiol 65:2912–2917
Charles LM, Eleftherios TP (1989) Increased levels of ATP and NADH are associated with increased solvent production in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 30:450–459
Pandit AV, Mahadevan R (2011) In silico characterization of microbial electrosynthesis for metabolic engineering of biochemicals. Microb Cell Fact 10:76
Girbal L, Soucaille P (1994) Regulation of Clostridium acetobutylicum metabolism as revealed by mixed-substrate steady-state continuous cultures: role of NADH/NAD ratio and ATP pool. J Bacteriol 176:6433–6438
Gheshlaghi R, Scharer JM, Moo-Young M, Chou CP (2009) Metabolic pathways of clostridia for producing butanol. Biotechnol Adv 27:764–781
Liu H, Grot S, Logan BE (2005) Electrochemically assisted microbial production of hydrogen from acetate. Environ Sci Technol 39:4317–4320
Acknowledgments
This work was supported by the “973” Program of China (Grant No. 2011CB707405), a Project Supported by Program for New Century Excellent Talents in University, Program for Changjiang Scholars and Innovative Research Team in University (Grant NO. 06-A-047), Jiangsu Province Natural Science Foundation for Youth (BK20140940), Jiangsu Province Natural Science Foundation (BK20151532), Jiangsu Key Lab of Biomass-based Green Fuels and Chemicals Foundation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
A.-Y. He and C.-Y. Yin contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
449_2015_1508_MOESM1_ESM.tif
Fig.S1 The cyclic voltammetry (CV) curve of ENR (0.1 mM NR) system at a scan rate of 20 mV/s over the range −300 to −1 mV during the batch adaptation of C. beijerinckii IB4 114 x 95 mm (96 x 96 DPI) (TIFF 127 kb)
Rights and permissions
About this article
Cite this article
He, AY., Yin, CY., Xu, H. et al. Enhanced butanol production in a microbial electrolysis cell by Clostridium beijerinckii IB4. Bioprocess Biosyst Eng 39, 245–254 (2016). https://doi.org/10.1007/s00449-015-1508-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1508-2