Abstract
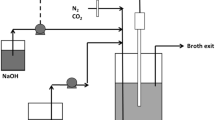
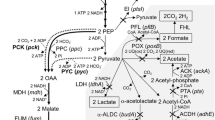
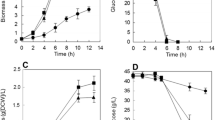
The effects of redox potential used as a control parameter on the process of succinic acid production in batch cultures of Actinobacillus succinogenes NJ113 have been investigated. In batch fermentation, cell growth and metabolite distribution were changed with redox potential levels in the range of −100 to −450 mV. From the results, the ORP level of −350 mV was preferable, which resulted in high succinic acid yield (1.28 mol mol−1), high succinic acid productivity (1.18 g L−1 h−1) and high mole ratio of succinic acid to acetic acid (2.02). The mechanism of redox potential regulation was discussed by metabolic flux analysis and the ratio of NADH/NAD+. We expected that redox potential can be used as a valuable parameter to monitor and control much more anaerobic fermentation production.




Similar content being viewed by others
References
Husson F, Tu VP, Santiago-Gomez M, Cachon R, Feron G, Nicaud JM, Kermasha S, Belin JM (2006) Effect of redox potential on the growth of Yarrowia lipolytica and the biosynthesis and activity of heterologous hydroperoxide lyase. J Mol Catal B Enzym 39:179–183
Ishizaki A, Snibai H, Hirose Y (1974) Basic aspects of electrode potential change in submerged fermentation. Agric Biol Chem 38:2399–2405
Berovic M (1999) Scale-up of citric acid fermentation by redox potential control. Biotechnol Bioeng 64:552–557
Radjai MK, Hatch RT, Cadman TW (1984) Optimisation of amino acid production by automatic self tuning digital control of redox potential. Biotechnol Bioeng Symp 14:657–666
Stolz P, Bocker V, Vogel RF, Hammes WP (1993) Utilisation of maltose and glucose by Lactobacilli isolated from sourdough. FEMS Microbiol Lett 109:237–242
Vonktaveesuk P, Tonokawa M, Ishizaki A (1994) Simulation of the rate of l-lactate fermentation using Lactoccocus lactis Io-1 by periodic electrodialysis. J Ferment Bioeng 77:508–512
Du CY, Yan H, Zhang YP, Li Y, Cao ZA (2006) Use of oxidoreduction potential as an indicator to regulate 1,3-propanediol fermentation by Klebsiella pneumoniae. Appl Microbiol Biotechnol 69:554–563
Bourel G, Hinnini S, Divies C, Garmyn D (2003) The response of Leuconostoc mesenteroides to low external oxidoreduction potential generated by hydrogen gas. J Appl Microbiol 94:280–288
Ouvry A, Wache Y, Tourdot-Marechal R, Divies C, Cachon R (2002) Effects of oxidoreduction potential combined with acetic acid, NaCl and temperature on the growth, acidification, and membrane properties of Lactobacillus plantarum. FEMS Microbiol Lett 214:257–261
Riondet C, Cachon R, Wache Y, Alcaraz G, Divies C (1999) Changes in the proton-motive force in Escherichia coli in response to external oxidoreduction potential. Eur J Biochem 262:595–599
Mazumdar S, Springs SL, McLendon GL (2003) Effect of redox potential of the heme on the peroxidase activity of cytochrome b562. Biophys Chem 105:263–268
Riondet C, Cachon R, Wache Y, Alcaraz G, Divies C (2000) Extracellular oxidoreduction potential modifies carbon and electron flow in Escherichia coli. J Bacteriol 182:620–626
Sridhar J, Eiteman MA (1999) Influence of redox potential on product distribution in Clostridium thermosuccinogenes. Appl Biochem Biotechnol 82:91–101
Sridhar J, Eiteman MA (2001) Metabolic flux analysis of Clostridium thermosuccinogenes—effects of pH and culture redox potential. Appl Biochem Biotechnol 94:51–69
Kastner JR, Eiteman MA, Lee SA (2003) Effect of redox potential on stationary-phase xylitol fermentations using Candida tropicalis. Appl Microbiol Biotechnol 63:96–100
Potera C (2005) Making succinate more successful. Environ Health Perspect 113:832–835
Zeikus JG, Jain MK, Elankovan P (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microb Biotechnol 51:545–552
Kim P, Laivenieks M, McKinlay J, Vieille C, Zeikus JG (2004) Construction of a shuttle vector for the overexpression of recombinant proteins in Actinobacillus succinogenes. Plasmid 51:108–115
Lee PC, Lee WG, Lee SY, Chang HN (1999) Succinic acid production by Anaerobiospirillum succiniciproducens: effects of the H2/CO2 supply and glucose concentration. Enzyme Microb Technol 24:549–554
Meynial-Salles I, Dorotyn S, Soucaille P (2008) A new process for the continuous production of succinic acid from glucose at high yield, titer, and productivity. Biotechnol Bioeng 99:129–135
Kim DY, Yim SC, Lee PC, Lee WG, Lee SY, Chang HN (2004) Batch and continuous fermentation of succinic acid from wood hydrolysate by Mannheimia succiniciproducens MBEL55E. Enzyme Microb Technol 35:648–653
Sanchez AM, Bennett GN, San KY (2005) Efficient succinic acid production from glucose through overexpression of pyruvate carboxylase in an Escherichia coli alcohol dehydrogenase and lactate dehydrogenase mutant. Biotechnol Progr 21:358–365
Wu H, Li ZM, Zhou L, Ye Q (2007) Improved succinic acid production in anaerobic culture of a pflB ldhA double mutant of Escherichia coli by enhancing the anaplerotic activities in preceding aerobic culture. Appl Environ Microbiol 73:7837–7843
Vemuri GN, Eiteman MA, Altman E (2002) Succinate production in dual-phase Escherichia coli fermentations depends on the time of transition from aerobic to anaerobic conditions. J Ind Microbiol Biotechnol 28:325–332
Guettler MV, Jain MK, Rumler D (1996) Method for making succinic acid, bacterial variants for use in the process, and methods for obtaining variants. US Patent 5,573,931
Song H, Lee SY (2006) Production of succinic acid by bacterial fermentation. Enzyme Microb Technol 39:352–361
Wimpenny JW (1969) The effect of Eh on regulatory processes in facultative anaerobes. Biotechnol Bioeng 11:623–629
Shibai H, Ichizaki A, Kobayashi K, Hirose Y (1974) Simultaneous measurement of dissolved oxygen and oxidation–reduction potentials in the aerobic culture. Agric Biol Chem 38:2407–2411
van der Werf MJ, Guettler MV, Jain MK, Zeikus JG (1997) Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch Microbiol 167:332–342
Park DH, Zeikus JG (1999) Utilization of electrically reduced neutral red by Actinobacillus succinogenes: physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J Bacteriol 181:2403–2410
McKinlay JB, Vieille C (2008) 13C-Metabolic flux analysis of Actinobacillus succinogenes fermentative metabolism at different NaHCO3 and H2 concentrations. Metab Eng 10:55–68
Chen KQ, Jiang M, Wei P, Yao JM, Wu H (2008) Succinic acid production from acid hydrolysate of corn fiber by Actinobacillus succinogenes. Appl Biochem Biotechnol. doi:10.1007/s12010-008-8367-0
Zhang YP, Huang ZH, Du CY, Li Y, Cao ZA (2009) Introduction of an NADH regeneration system into Klebsiella oxytoca leads to an enhanced oxidative and reductive metabolism of glycerol. Metab Eng 11:101–106
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Lin H, Bennett GN, San KY (2005) Fed-batch culture of a metabolically engineered Escherichia coli strain designed for high-level succinate production and yield under aerobic conditions. Biotechnol Bioeng 90:775–779
Menzel K, Ahrens K, Zeng AP, Deckwer WD (1998) Kinetic, dynamic, and pathway studies of glycerol metabolism by Klebsiella pneumoniae in anaerobic continuous culture: IV. Enzymes and fluxes of pyruvate metabolism. Biotechnol Bioeng 60:617–626
Acknowledgments
This work was supported by grant 2009CB724701 from “973” Program of china, Grant No. 20606017 from National Natural Science Foundation of China, and Qing Lan Project of Jiangsu province.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Metabolic reactions:
(rBM) 0.0102 G6P + 0.0032 F6P +0.0364 GAP + 0.0131 PEP + 0.0685 PYR + 0.0746 ACA + 0.0372 OAA + 0.0154 R5P + 0.0077 E4P + 1.1640 ATP + 0.3334 NADPH = Biomass + 1.1640 ADP + 0.3334 NADP.
Rights and permissions
About this article
Cite this article
Li, J., Jiang, M., Chen, KQ. et al. Effect of redox potential regulation on succinic acid production by Actinobacillus succinogenes . Bioprocess Biosyst Eng 33, 911–920 (2010). https://doi.org/10.1007/s00449-010-0414-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-010-0414-x