Abstract
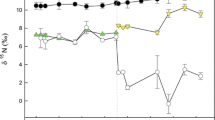
Food web studies based on stable C and N isotope ratios usually assume isotopic equilibrium between a consumer and its diet. In the Arctic, strong seasonality in food availability often leads to diet switching, resulting in a consumer’s isotopic composition to be in flux between different food sources. Experimental work investigating the time course and dynamics of isotopic change in Arctic fauna has been lacking, although these data are crucial for accurate interpretation of food web relationships. We investigated seasonal (ice-covered spring vs. ice-free summer) and temperature (1 vs. 4°C) effects on growth and stable C and N isotopic change in the common nearshore Arctic amphipod Onisimus litoralis following a diet switch and while fasting in the laboratory. In spring we found no significant temperature effect on N turnover [half-life (HL) estimates: HL-N = 20.4 at 4°C, 22.4 days at 1°C] and a nonsignificant trend for faster growth and C turnover at the higher temperature (HL-C = 13.9 at 4°C, 18.7 days at 1°C). A strong seasonal effect was found, with significantly slower growth and C and N turnover in the ice-free summer period (HL-N = 115.5 days, HL-C = 77.0 days). Contrary to previous studies, metabolic processes rather than growth accounted for most of the change in C and N isotopic composition (84–89 and 67–77%, respectively). This study provides the first isotopic change and metabolic turnover rates for an Arctic marine invertebrate and demonstrates the risk of generalizing turnover rates based on taxon, physiology, and environment. Our results highlight the importance of experimental work to determine turnover rates for species of interest.




Similar content being viewed by others
References
Arndt CE, Beuchel F (2006) Life history and population dynamics of the Arctic sympagic amphipods Onisimus nanseni Sars and O. glacialis Sars (Gammaridea: Lysianassidae). Polar Biol 29:239–248
Attkinson A, Meyer B, Stübing D, Hagen W, Schmidt K, Bathmann UV (2002) Feeding and energy budgets of Antarctic krill Euphausia superba at the onset of winter. II. Juveniles and adults. Mar Ecol Prog Ser 160:63–76
Bluhm BA, Gradinger R (2008) Regional variability in food availability for Arctic marine mammals. Ecol Appl 18[Suppl]:77–96
Bosley KL, Witting DA, Chambers RC, Wainright SC (2002) Estimating turnover rates of carbon and nitrogen in recently metamorphosed winter flounder Pseudopleuronectes americanus with stable isotopes. Mar Ecol Prog Ser 236:233–240
Boudrias MA, Carey AG Jr (1988) Life history patterns of Pseudolibrotus litoralis (Crustacea: Amphipoda) on the inner continental shelf, SW Beaufort Sea. Mar Ecol Prog Ser 49:249–257
Bradstreet SW, Cross WE (1982) Trophic relationships at high ice edges. Arctic 35:1–12
Carey AG Jr (1992) The ice fauna in the shallow southwestern Beaufort Sea, Arctic Ocean. J Mar Syst 3:225–236
Carey AG Jr, Boudrias MA (1987) Feeding ecology of Pseudolibrotus (=Onisimus) litoralis Kröyer (Crustacea: Amphipoda) on the Beaufort Sea inner continental shelf. Polar Biol 8:29–33
Chapelle G, Peck LS (1995) The influence of acclimation and substratum on the metabolism of the Antarctic amphipods Waldeckia obesa (Chevreux 1905) and Bovallia gigantea (Pfeffer 1888). Polar Biol 15:225–232
Collie JS (1985) Life history and production of three amphipod species on Georges Bank. Mar Ecol Prog Ser 22:229–238
Dower KM, Lucas MI, Phillips R, Dieckmann G, Robinson DH (1996) Phytoplankton biomass, P–I relationships and primary production in the Weddell Sea, Antarctica, during the austral autumn. Polar Biol 16:41–52
Frazer TK (1996) Stable isotopes composition (δ13C and δ15N) of larval krill, Euphausia superba, and two of its potential food sources in winter. J Plankton Res 18:1413–1426
Frazer TK, Ross RM, Quentin LB, Montoya JP (1997) Turnover of carbon and nitrogen during growth of larval krill, Euphausia superba Dana: a stable isotope approach. J Exp. Mar Biol Ecol 212:259–257
Fry B, Arnold C (1982) Rapid 13C/12C turnover during growth of brown shrimp (Panaeus aztecus). Oecologia 54:200–204
Gannes LZ, O’Brien DM, Del Rio CM (1997) Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78:1271–1276
Gorokhova E, Hansson S (1999) An experimental study on variations in stable carbon and nitrogen isotope fractionation during growth of Mysis mixta and Neomysis integer. Can J Fish Aquat Sci 56:2203–2210
Gradinger RR (2002) Sea ice microorganisms. In: Bitten G (ed) Encyclopedia of environmental microbiology. Wiley, Hoboken, N.J., pp 2833–2844
Gradinger R (2008) Sea ice algae: major contributors to primary production and algal biomass in the Chukchi and Beaufort Sea. Deep Sea Res (in press)
Gradinger RR, Bluhm BA (2004) In situ observations on the distribution and behavior of amphipods and Arctic cod (Boreogadus saida) under the sea ice of the high Arctic Canadian Basin. Polar Biol 27:595–603
Gradinger RR, Bluhm BA (2005) Susceptibility of sea ice biota to disturbances in the shallow Beaufort Sea. Phase 1: Biological coupling of sea ice with the pelagic and benthic realms. Final report, OCS Study MMS 2005–062
Graeve M, Kattner G, Piepenburg D (1997) Lipids in Arctic benthos: does the fatty acid and alcohol composition reflect feeding and trophic interactions? Polar Biol 18:53–61
Haddon M (2001) Modeling and quantitative methods in fisheries. Chapman and Hall, Boca Raton
Herzka SZ, Holt JG (2000) Changes in isotopic composition of red drum (Sciaenops ocellatus) larvae in response to dietary shifts: potential applications to settlement studies. Can J Fish Aquat Sci 57:137–147
Hesslein RH, Hallard KA, Ramlal P (1993) Replacement of sulfur, carbon and nitrogen in tissue of the growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C and δ15N. Can J Fish Aquat Sci 50:2071–2076
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188
Hobson KA, Welch HE (1992) Determination of trophic relationships within a high Arctic marine food web using delta-13°C and delta-15 N analysis. Mar Ecol Prog Ser 84:9–18
Hobson KA, Alisauskas RT, Clark RG (1993) Stable isotope enrichment in avian tissues due to fasting and nutritional stress: implications for isotopic analysis of diet. Condor 95:388–394
Hochachka P, Somero G (2002) Biochemical adaptation, mechanism and physical evolution. Oxford University Press, New York
Horner RS, Ackley F, Dieckmann GS, Gulliksen B, Hoshiai T, Legendre L, Melnikov IA, Reeburgh WS, Spindler M, Sullivan CW (1992) Ecology of sea ice biota, 1: Habitat, terminology, and methodology. Polar Biol 12:417–427
Horner R (1985) Sea ice biota. CRC, Boca Raton
Iken K, Bluhm BA, Gradinger R (2005) Food web structure in the high Arctic Canada Basin: evidence from δ13C and δ15N analysis. Polar Biol 28:238–249
Jardine TD, MacLatchy DL, Fairchild WL, Cunjak RA, Brown SB (2004) Rapid carbon turnover during growth of Atlantic Salmon (Salmo salar) smolts in sea water, and evidence for reduced food consumption by growth-stunts. Hydrobiologia 527:63–73
Lovvorn JR, Cooper LW, Brooks ML, De Ruyck CC, Bump JK, Grebmeier JM (2005) Organic matter pathways to zooplankton and benthos under pack ice in late winter and open water in late summer in the north-central Bering Sea. Mar Ecol Prog Ser 291:135–150
MacAvoy SE, Macko SA, Garman GC (2001) Isotopic turnover in aquatic predators: quantifying the exploitation of migratory prey. Can J Fish Aquat Sci 58:923–932
MacAvoy SE, Macko SA, Arneson LS (2005) Growth versus metabolic tissue replacement in mouse tissues determined by stable carbon and nitrogen isotope analysis. Can J Zool 83:631–641
Maruyama M, Yamada Y, Rusuwa B, Yuma M (2001) Change in stable nitrogen isotope ratio in the muscle tissue of a migratory goby, Rhinogobius sp., in a natural setting. Can J Fish Aquat Sci 58:2125–2128
McCutchan JH Jr, Lewis WM Jr, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
McIntyre PB, Flecker AS (2006) Rapid turnover of tissue nitrogen in primary consumers in tropical freshwaters. Oecologia 148:12–21
McMahon KW, Ambrose WG, Johnson BJ, Sun M, Lopez GR, Clough LM, Carroll ML (2006) Benthic community response to ice algae and phytoplankton in Ny Alesund, Svalbard. Mar Ecol Prog Ser 310:1–14
McWhinnie MA (1964) Temperature responses and tissue respiration in Antarctic crustacea with particular reference to the krill, Euphaasia superba. Antarct Res Ser 1:63–72
Peck L (2002) Ecophysiology of Antarctic marine ectotherms: limits to life. Polar Biol 25:31–40
Percy JA (1993) Energy consumption and metabolism during starvation in the Arctic hyperiid amphipod Themisto libellula. Polar Biol 13:549–555
Percy JA, Fife FJ (1981) The biochemical composition and energy content of Arctic marine macrozooplankton. Arctic 34:307–313
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Rakusa-Suszczewski S (1972) The biology of Paramoera walkeri Stebbing (amphipoda) and the antarctic sub-fast ice community. Polar Arch Hydrobiol 19:11–36
Robertson RF, El-Haj AJ, Peck LS, Taylor EW (2001) The effects of temperature on metabolic rate and protein synthesis following a meal in the isopod Glyptonotus antarcticus Eights (1852). Polar Biol 24:677–686
Sainte-Marie B (1986) Foraging by lysianassid amphipods. PhD thesis, Dalhousie University, Halifax
Sainte-Marie B, Percy JA, Shea JR (1989) A comparison of meal size and feeding rate of the lysianassid amphipods Anonyx nugax, Onisimus (= Pseudalibrotus) litoralis and Orchomenella pinguis. Mar Biol 102:361–368
Sakano H, Fujiwara E, Nohara S, Ueda H (2005) Estimation of nitrogen stable isotope turnover rate of Onchorynchus nerka. Environ Biol Fishes 72:13–18
Sakshaug E (2004) Primary and secondary production in the Arctic seas. In: Stein R, Macdonald RW (eds) The organic carbon cycle in the Arctic Ocean. Springer, New York, pp 57–81
Schmidt K, Atkinson A, Stübing D, McClelland JW, Montoya JP, Voss M (2003) Trophic relationships among Southern Ocean copepods and krill: some uses and limitations of a stable isotope approach. Limnol Oceanogr 48:277–289
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment. Cambridge University Press, Cambridge
Suzuki KW, Kasai A, Nakayama K, Tanaka M (2005) Differential isotopic enrichment and half-life among tissues in Japanese temperate bass (Lateolabrax japonicus) juveniles: implications for analyzing migration. Can J Fish Aquat Sci 62:671–678
Tamelander T, Renaud PE, Hop H, Carroll ML, Ambrose WG, Hobson KA (2006) Trophic relationships and pelagic–benthic coupling during summer in the Barents Sea marginal ice zone, revealed by stable carbon and nitrogen isotope measurements. Mar Ecol Prog Ser 310:33–46
Tieszen LL, Boutton TW, TeSDahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37
Tominaga O, Ono N, Seikai T (2003) Influence of diet shift from formulated feed to live mysids on the carbon and nitrogen stable isotope ratio (δ13C and δ15N) in dorsal muscles of juvenile Japanese flounders, Paralichthys olivaceus. Aquaculture 218:265–276
Weingartner TJ, Okkonen SR, Danielson SL (2005) Circulation and water property variations in the nearshore Alaskan Beaufort Sea. Final report. OCS study MMS 2005–028
Werner I (2000) Faecal pellet production by Arctic under-ice amphipods—transfer of organic matter through the ice/water interface. Hydrobiologia 426:89–96
Werner I, Auel H (2005) Seasonal variability in abundance, respiration and lipid composition of under ice amphipods. Mar Ecol Prog Ser 292:251–262
Witting DA, Chambers RC, Bosley KL, Wainright SC (2004) Experimental evaluation of ontogenetic diet transitions in summer flounder (Paralichthys dentatus), using stable isotopes as diet tracers. Can J Aquat Sci 61:2069–2084
Yokoyama H, Tamaki A, Harada K, Shimoda K, Koyama K, Ishihi K (2005) Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Mar Ecol Prog Ser 296:115–128
Acknowledgements
This publication is the result of research sponsored by Alaska Sea Grant with funds from the National Oceanic and Atmospheric Administration Office of Sea Grant, Department of Commerce under grant no. NA 16RG2321 (project no. R/101-04) and from the University of Alaska with funds appropriated by the state. Additional funding was provided by the Center for Global Change. We want to thank S. Sugai for her special attention and interest in the successful completion of this project. We are grateful to the staff of the Barrow Arctic Science Consortium who greatly simplified field logistics and to S. Story, S. Lee, K. Meiners, and M. Kaufman for their field assistance. T. Howe and N. Haubenstock at the Alaska Stable Isotope Facility analyzed the many stable isotope samples collected for this research. We also want to thank the School of Fisheries and Ocean Sciences and its office staff, with special thanks to C. Neumann, for their support and assistance. M. Castellini and M. Wooller are thanked for their very helpful suggestions and valuable contributions to the Master’s thesis by M. Nielson-Kaufman, which is the basis for this manuscript. The experiments conducted during this study were in compliance with current United States laws governing ethical conduct and the care and use of animals in research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marc Mangel.
Rights and permissions
About this article
Cite this article
Kaufman, M.R., Gradinger, R.R., Bluhm, B.A. et al. Using stable isotopes to assess carbon and nitrogen turnover in the Arctic sympagic amphipod Onisimus litoralis . Oecologia 158, 11–22 (2008). https://doi.org/10.1007/s00442-008-1122-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1122-y