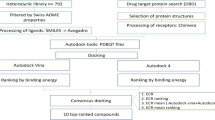
Abstract
Malaria, caused by protozoa of the genus Plasmodium, is a disease that infects hundreds of millions of people annually, causing an enormous social burden in many developing countries. Since current antimalarial drugs are starting to face resistance by the parasite, the development of new therapeutic options has been prompted. The enzyme Plasmodium falciparum enoyl-ACP reductase (PfENR) has a determinant role in the fatty acid biosynthesis of this parasite and is absent in humans, making it an ideal target for new antimalarial drugs. In this sense, the present study aimed at evaluating the in silico binding affinity of natural and synthetic amides through molecular docking, in addition to their in vitro activity against P. falciparum by means of the SYBR Green Fluorescence Assay. The in vitro results revealed that the natural amide piplartine (1a) presented partial antiplasmodial activity (20.54 μM), whereas its synthetic derivatives (1m—IC50 104.45 μM), (1b, 1g, 1k, and 14f) and the natural amide piperine (18a) were shown to be inactive (IC50 > 200 μM). The in silico physicochemical analyses demonstrated that compounds 1m and 14f violated the Lipinski's rule of five. The in silico analyses showed that 14f presented the best binding affinity (− 13.047 kcal/mol) to PfENR and was also superior to the reference inhibitor triclosan (− 7.806 kcal/mol). In conclusion, we found that the structural modifications in 1a caused a significant decrease in antiplasmodial activity. Therefore, new modifications are encouraged in order to improve the activity observed.
Similar content being viewed by others
Change history
21 May 2020
The original version of the article above contained an error in the text.
References
Alexandre MA, Ferreira CO, Siqueira AM, Magalhães BL, Mourão MPG, Lacerda MV, Alecrim MDGC (2010) Severe plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis 16(10):1611–1614
Amir A, Cheong FW, de Silva JR, Liew JWK, Lau YL (2018) Plasmodium knowlesi malaria: current research perspectives. Infect Drug Resist 11:1145–1155
Araújo-Júnior JX, da Cunha EVL, Chaves MCO, Alexander IG (1997) Piperdardine, a piperidine alkaloid from Piper tuberculatum. Phytochemistry 44:559–561
Araújo-Vilges KM, de Oliveira SV, Couto SCP, Fokoue HH, Romero GAS, Kato MJ, Romeiro LAS, Leite JRSA, Kuckelhau SAS (2017) Effect of piplartine and cinnamides on Leishmania amazonensis, Plasmodium falciparum and on peritoneal cells of Swiss mice. Pharm Biol 55:601–1607
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien N, Thanh NV, Phu NH, Htut Y, Han K, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, Macinnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WR, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ (2014) Spread of artemisinin resistance in Plasmodium falciparum malaria. New Eng J Med 371:411–423
Bezerra DP, de Castro FO, Alves AP, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, de Alencar NM, Mesquita RO, Lima MW, Costa-Lotufo LV (2008) In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J Appl Toxicol 28:156–163
Bezerra DP, Pessoa C, Moraes MO, Saker-Neto N, Silveira ER, Costa Lotufo LV (2013) Overview of the therapeutic potential of piplartine (Piperlongumine). Eur J Pharm Sci 48:453–463
Bodiwala HS, Singh G, Singh R, Singh CS, Dey SS, Sharma KK, Bhutani IP (2007) Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J Nat Med 61:418–421
Boechat N, Ferreira MDEL, Pinheiro LC, Jesus AM, Leite MM, Júnior CC, Aguiar AC, de Andrade IM, Krettli AU (2014) New compounds hybrids 1h-1,2,3-triazole-quinoline against Plasmodium falciparum. Chem Biol Drug Des 84:325–332
Calvo-Calle JM, Moreno A, Eling WM, Nardin EH (1994) In vitro development of infectious liver stages of P. yoelii and P. berghei malaria in human cell lines. Exp Parasitol 79:362–373
Campelo Y, Ombredane A, Vasconcelos AG, Albuquerque L, Moreira DC, Plácido A, Rocha J, Fokoue HH, Yamaguchi L, Mafud A, Mascarenhas YP, Delerue-Matos C, Borges T, Joanitti GA, Arcanjo D, Kato MJ, Kuckelhaus S, Silva M, Moraes J, Leite JRSA (2018) Structure-activity relationship of piplartine and synthetic against Schistosoma mansoni and cytotoxicity to mammalian cells. Int J Mol Sci 19:1802–1818
Cotinguiba F, Regasini LO, Bolzani VS, Debonsi HM, Passerini GD, Sicarelli RMB, Kato MJ, Furlan M (2009) Piperamides and their derivatives as potential anti-trypanosomal agents. Med Chem Res 18:703–711
Cox FE (2010) History of the discovery of the malaria parasites and their vectors. Parasit Vectors 3:5–13
Fokoue HH (2015) Síntese, atividades biológicas e estudo de relação de estrutura-atividade de piperamidas. Doctoral thesis. Univerisity of São Paulo
Fokoue HH, Marques JV, Correia MV, Yamaguchi LFXQU, Aires-de-Sousa J, Scotti MT, Lopes NP, Kato MJ (2018) Fragmentation pattern of amides by EI and HRESI: study of protonation sites using DFT-3LYP data. RSC Adv 8:21407–21413
Freitas RP (2015) Avaliação da atividade esquistossomicida de análogos sintéticos da piplartina em vermes adultos de Schistosoma mansoni. Dissertação de Mestrado apresentada ao Programa de Pós-Graduação Interunidades em Biotecnologia USP/IPT/Instituto Butantan. USP
Gomes PR, Miguel FB, de Oliveira ME, Ferreira VV, Guimarães DSM, de Lima AB, Barbosa CS, de Oliveira MA, de Almeida MV, Viana GHR, Couri MRC, Varotti FP (2014) Síntese e avaliação da atividade antimalárica de compostos derivados da curcumina. Quim Nova 37(3):492–496
Guerra TM (2019) Estudos de docking molecular de derivados da tiazolidina como potenciais inibidores da enzima cruzaína de Trypanosoma cruzi. Dissertation. Federal Rural University of Pernambuco
Gutiérrez RMP, Gonzalez AMN, Hoyo-Vadillo C (2013) Alkaloids from piper: a review of its phytochemistry and pharmacology. Mini Rev Med Chem 13:163–193
Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW (2010) Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med 7:e1000290. https://doi.org/10.1371/journal.pmed.1000290
Katsuno K, Burrows JN, Duncan K, van Huijsduijnen RH, Kaneko T, Kita K, Mowbray CE, Schmatz D, Warner P, Slingsby BT (2015) Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 14:751–758
Krafts K, Hempelmann E, Skórska-Stania A (2012) From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy. Parasitol Res 111:1–6
Lambros C, Vanderberg J (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol:418–420
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341
Mackintosh CL, Beeson JG, Marsh K (2004) Clinical features and pathogenesis of severe malaria. Trend Parasitol 20:597–603
Magalhães UO (2009) Modelagem Molecular e Avaliação da Relação Estrutura-Atividade Acoplados a Estudos Físico-Químicos, Farmacocinéticos e Toxicológicos In Silico de Derivados Heterocíclicos com Atividade Leishmanicida. Dissertation. Federal University of Rio de Janeiro
Moreira F, Riul T, Moreira M, Pilon A, Dias-Baruffi M, Araújo M, Lopes N, de Oliveira A (2018) Leishmanicidal Effects of Piperlongumine (Piplartine) and Its Putative Metabolites. Planta Med 84:1141–1114
Mossmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nogueira F, Rosário E (2010) Métodos para avaliação da atividade antimalárica nas diferentes fases do ciclo de vida do Plasmodium Methods. Revista Pan-Amaz Saúde 1:109–124.v.1 n.3 Ananindeua set. 2010. https://doi.org/10.5123/S2176-62232010000300015
Oliveira CS (2013) Síntese, caracterização e atividade antimicrobiana de compostos heterocíclicos da classe 2,3-diidro-1,3,4-oxadiazol derivados de N-acilhidrazonas. Doctoral thesis. Federal Univesity of Paraíba
Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OM, Prasad AK, Wengel J, Olsen CE, Boll PM (1997) Phytochemistry of the genus Piper. Phytochemistry 46:597–673
Piccirillo E, Amaral AT (2018) Busca virtual de compostos bioativos: conceitos e aplicações. Quím Nova, São Paulo 41:662–677
Pidugu LS, Kapoor M, Surolia N, Surolia A, Suguna K (2004) Structural basis for the variation in triclosan affinity to enoyl reductases. J Mol Biol 343:147–155
Ponte-Sucre A, Bruhn H, Schirmeister T, Cecil A, Albert CR, Buechold C, Tischer M, Schlesinger S, Goebel T, Fuß A, Mathein D, Merget B, Sotriffer CA, Stich A, Krohne G, Engstler M, Bringmann G, Holzgrabe U (2015) Anti-trypanosomal activities and structural chemical properties of selected compound classes. Parasitol Res 114:501–512
Qidwai T, Khan F (2012) Antimalarial drugs and drug targets specific to fatty acid product discovery. Chem Biol Drug Des 80(2):155–172. https://doi.org/10.1111/j.1747-0285.2012.01389.x
Ryckmans T, Edwards MP, Horne VA, Correia AM, Owen DR, Thompson LR, Tran I, Tutt MF, Young T (2009) Rapid assessment of a novel series of selective CB2 agonists using parallel synthesis protocols: a lipophilic efficiency (LipE) analysis. Bioorg Med Chem Lett 19:4406–4409
Schrödinger; SCHRÖDINGER RELEASE (2015–2): LigPrep, version 3.4; Schrödinger, LLC, New York, 2015a
Schrödinger; SCHRÖDINGER RELEASE(2015–2): Maestro, version 10.2.010; Schrödinger, LLC, New York, 2015b
Schrödinger; SCHRÖDINGER RELEASE (2015–2): Schrödinger Suite 2015–2 Protein Preparation Wizard; Epik version 3.2; Schrödinger, LLC, New York, NY, 2015; Impact version 6.7, Schrödinger, LLC, New York, NY, 2015; Prime version 4.0, Schrödinger, LLC, New York, NY, 2015c
Schrödinger; Small-Molecule Drug Discovery Suite(2015–2): Schrödinger Suite 2015–2 Induced Fit Docking protocol; Glide version 6.7, Schrödinger, LLC, New York, NY, 2015; Prime version 4.0; Schrödinger, LLC, New York, NY, 2015
Silva-Jardim I, Horta MF, Ramalho-Pinto FJ (2004) The Leishmania chagasi proteasome: role in promastigotes growth and amastigotes survival within murine macrophages. Acta Trop 91:121–130
Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806
Tasdemir D (2006) Type II fatty acid biosynthesis, a new approach in antimalarial natural metabolic pathway of Plasmodium falciparum. Phytochem Rev 5:99–108. https://doi.org/10.1007/s11101-005-5297-0
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem Jun 45:2615–2623
WHO, World Malaria Report (2018) Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. http://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1. Acess 29 November
Wink M (2012) Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules 17:12771–12791
Zubairi ABS, Nizami S, Raza A, Mehraj V, Rasheed AF, Ghanchi NK et al (2013) Severe Plasmodium vivax malaria in Pakistan. Emerg Infect Dis 19:1851
Acknowledgments
We are thankful to the Instituto Federal de Rondônia, campus of Porto Velho – Calama; Plataforma de Bioensaios de Malária e Leishmaniose – FIOCRUZ-RO; Institute of Chemistry of the Federal University of São Paulo – USP; the Instituto Nacional de Ciência e Tecnologia em Fármacos e Medicamentos (INCT-INOFAR), Rede Mineira de Química (RQ-MG), FAPEMIG and CNPq; and Programa de Pós-Graduação em Biologia Experimental – PGBIOEXP/UNIR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Tobili Sam-Yellowe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1.16 MB)
Rights and permissions
About this article
Cite this article
da Silva, M.A., Veloso, M.P., de Souza Reis, K. et al. In silico evaluation and in vitro growth inhibition of Plasmodium falciparum by natural amides and synthetic analogs. Parasitol Res 119, 1879–1887 (2020). https://doi.org/10.1007/s00436-020-06681-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06681-9