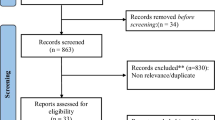
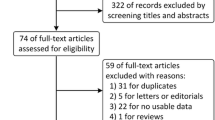
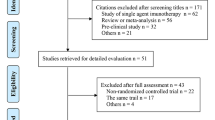
Abstract
Aims
Single-agent anti-PD-1/PD-L1 clinical efficacy against < 1% PD-L1-expressing non-small-cell lung cancers (NSCLCs) is controversial.
Methods
This meta-analysis examined randomized-trial data comparing first-line PD-1/PD-L1-inhibitor + chemotherapy (CT) vs CT alone for advanced < 1% PD-L1 NSCLCs. Outcome measures included overall survival (OS), progression-free survival (PFS) and objective response rate (ORR).
Results
IMpower (atezolizumab + CT), Keynote (pembrolizumab + CT) and CheckMate (nivolumab + CT) trials included 2037 NSCLCs (1246 PD-L1–negative; 791 < 1% PD-L1 expression). Anti-PD-1/PD-L1 + CT was significantly associated (hazard ratio [95% confidence interval]) with prolonged OS (0.75 [0.63–0.89]; p = 0.0008) and PFS (0.72 [0.65–0.80]; p < 0.0001), and higher ORR (odds ratio 2.06 [1.50–2.83]; p < 0.0001).
Conclusions
First-line anti-PD-1/PD-L1 + CT combination appears superior to CT alone for advanced, < 1% PD-L1-expressing NSCLCs for OS, PFS and ORR.







Similar content being viewed by others
Abbreviations
- ALK:
-
Anaplastic lymphoma kinase
- ChT:
-
Chemotherapy
- CI:
-
Confidence interval
- DOR:
-
Duration of response
- EGFR:
-
Epidermal growth factor receptor
- HR:
-
Hazard ratio
- ICI:
-
Immune-checkpoint inhibitor
- NSCLC:
-
Non-small-cell lung cancer
- Nsq:
-
Non-squamous cell
- OR:
-
Odds ratio
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-1:
-
Programmed cell-death protein-1
- PD-L1:
-
Programmed cell-death protein-1 ligand
- PFS:
-
Progression-free survival
- Sq:
-
Squamous cell
References
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Borghaei H, Hellmann MD, Paz-Ares LG, Ramalingam SS, Reck M, O’Byrne KJ et al (2018) Nivolumab (Nivo) + platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with < 1% tumor PD-L1 expression: results from CheckMate 227. J Clin Oncol. 36(15_suppl):9001
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135
Cappuzzo F, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ et al (2018) LBA53IMpower130: progression-free survival (PFS) and safety analysis from a randomised phase III study of carboplatin + nab-paclitaxel (CnP) with or without atezolizumab (atezo) as first-line (1L) therapy in advanced non-squamous NSCLC. Ann Oncol. https://doi.org/10.1093/annonc/mdy424.065
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M et al (2017) First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 376(25):2415–2426
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 378:2078–2092
Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM (2019) Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol 30(2):219–235
Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C et al (2018) Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378:2093–2104
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet Lond Engl 387(10027):1540–1550
Jotte RM, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez Abreu D, Hussein MA et al (2018) IMpower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 36(18):LBA9000
Landre T, Des Guetz G, Vergnenegre A, Chouaid C (2019) 165PClinical benefit of anti PD-1/PD-L1 plus chemotherapy versus chemotherapy alone in first-line treatment in advanced non-small cell lung cancer: a meta-analysis. Ann Oncol. https://doi.org/10.1093/annonc/mdz063.063/5445436
Mok T, Johnson M, Garon E, Peters S, Soria J, Wang L et al (2017) P1.04–008 POSEIDON: a phase 3 study of first-line durvalumab ± tremelimumab + chemotherapy vs chemotherapy alone in metastatic NSCLC. J Thorac Oncol 12(11):S1975
Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet Lond Engl 393(10183):1819–1830
Papadimitrakopoulou V, Gadgeel SM, Borghaei H, Gandhi L, Patnaik A, Powell SF et al (2017) First-line carboplatin and pemetrexed (CP) with or without pembrolizumab (pembro) for advanced nonsquamous NSCLC: Updated results of KEYNOTE-021 cohort G. J Clin Oncol 35(15_suppl):9094
Papadimitrakopoulou V, Cobo M, Bordoni R, Dubray-Longeras P, Szalai Z, Ursol G et al (2018) OA05.07 IMpower132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol. 13(10):S332–S333
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet Lond Engl 389(10066):255–265
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301
Wang X-J, Lin J-Z, Yu S-H, Wu S-X, Luo H-S, Du Z-S et al (2019) First-line checkpoint inhibitors for wild-type advanced non-small-cell cancer: a pair-wise and network meta-analysis. Immunotherapy 11(4):311–320
Zhou Y, Chen C, Zhang X, Fu S, Xue C, Ma Y et al (2018) Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. J Immunother Cancer 6(1):155
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Landre, T., Des Guetz, G., Chouahnia, K. et al. First-line PD-1/PD-L1 inhibitor plus chemotherapy vs chemotherapy alone for negative or < 1% PD-L1-expressing metastatic non-small-cell lung cancers. J Cancer Res Clin Oncol 146, 441–448 (2020). https://doi.org/10.1007/s00432-019-03070-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-03070-3