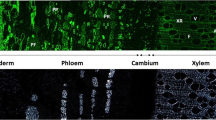
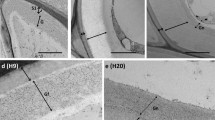
Abstract
Xylans occupy approximately one-third of the cell wall components in hardwoods and their chemical structures are well understood. However, the microdistribution of xylans (O-acetyl-4-O-methylglucuronoxylans, AcGXs) in the cell wall and their correlation with functional properties of cells in hardwood xylem is poorly understood. We demonstrate here the spatial and temporal distribution of xylans in secondary xylem cells of hybrid aspen using immunolocalization with LM10 and LM11 antibodies. Xylan labeling was detected earliest in fibers at the cell corner of the S1 layer, and then later in vessels and ray cells respectively. Fibers showed a heterogeneous labeling pattern in the mature cell wall with stronger labeling of low substituted xylans (lsAcGXs) in the outer than inner cell wall. In contrast, vessels showed uniform labeling in the mature cell wall with stronger labeling of lsAcGXs than fibers. Xylan labeling in ray cells was detected much later than that in fibers and vessels, but was also detected at the beginning of secondary cell wall formation as in fibers and vessels with uniform labeling in the cell wall regardless of developmental stage. Interestingly, pit membranes including fiber–, vessel– and ray–vessel pits showed strong labeling of highly substituted xylans (hsAcGXs) during differentiation, although this labeling gradually disappeared during pit maturation. Together our observations indicate that there are temporal and spatial variations of xylan deposition and chemical structure of xylans between cells in aspen xylem. Differences in xylan localization between aspen (hardwood) and cedar (softwood) are also discussed.










Similar content being viewed by others
Abbreviations
- AGXs:
-
Arabino-4-O-methylglucuronoxylans
- G lignin:
-
Guaiacyl lignin
- hsAcGXs:
-
Highly substituted O-acetyl-4-O-methylglucuronoxylans
- IL:
-
Intercellular layer
- lsAcGXs:
-
Low substituted O-acetyl-4-O-methylglucuronoxylans
- MLcc:
-
Middle lamella cell corner
- PL:
-
Protective layer
- PW:
-
Primary cell wall
- S lignin:
-
Syringyl lignin
References
Atalla R (2005) The role of the hemicelluloses in the nanobiology of wood cell walls: a systems theoretic perspective. In: proceedings of the hemicelluloses workshop 2005. University of Canterbury, Christchurch, pp 37–57
Awano T, Takabe K, Fujita M (1998) Localization of glucuronoxylans in Japanese beech visualized by immunogold labeling. Protoplasma 202:213–222
Awano T, Takabe K, Fujita M, Daniel G (2000) Deposition of glucuronoxylans on the secondary cell wall of Japanese beech as observed by immuno-scanning electron microscopy. Protoplasma 212:72–79
Awano T, Takabe K, Fujita M (2002) Xylan deposition on secondary wall of Fagus crenata fiber. Protoplasma 219:106–115
Barnett JR, Cooper P, Bonner LJ (1993) The protective layer as an extension of the apoplast. IAWA J 14:163–171
Chafe SC (1974) Cell wall formation and “protective layer” development in the xylem parenchyma of trembling aspen. Protoplasma 80:335–354
Daniel G, Nilsson T, Pettersson B (1991) Poorly and non-lignified regions in the middle lamella cell corners of birch (Betula verrucosa) and other wood species. IAWA Bull n s 12:70–83
Donaldson LA (2001) Lignification and lignin topochemistry—an ultrastructural view. Phytochemistry 57:859–873
Fengel D, Wegener G (1989) Wood: chemistry, ultrastructure, reactions. de Gruyter, Berlin
Filonova L, Gunnarsson LC, Daniel G, Ohlin M (2007) Synthetic xylan-binding modules for mapping of pulp fibres and wood sections. BMC Plant Biol 7:54–64
Grünwald C, Ruel K, Schmitt U (2002) On the cytochemistry of cell wall formation in poplar trees. Plant Biol 4:13–21
Hervé C, Rogowski A, Gilbert HJ, Knox JP (2009) Enzymatic treatments reveal differential capacities for xylan recognition and degradation in primary and secondary plant cell walls. Plant J 58:413–422
Ilvessalo-Pfāffli MS (1994) Fiber atlas: identification of papermaking fibers. Springer-Verlag, Berlin
Kaneda M, Rensing K, Samuels L (2010) Secondary cell wall deposition in developing secondary xylem of polpar. J Integr Plant Biol 52:234–243
Kim JS, Awano T, Yoshinaga A, Takabe K (2010) Imunolocalization and structural variations of xylan in differentiating earlywood tracheids cell walls of Cryptomeria japonica. Planta 232:817–824
Kim JS, Awano T, Yoshinaga A, Takabe K (2011) Temporal and spatial diversities of the immunolabeling of mannan and xylan polysaccharides in differentiating earlywood ray cells and pits of Cryptomeria japonica. Planta 233:109–122
McCartney L, Marcus SE, Knox JP (2005) Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J Histochem Cytochem 53:543–546
Mellerowicz EJ, Baucher M, Sundberg B, Boerjan W (2001) Unravelling cell wall formation in the woody dicot stem. Plant Mol Biol 47:239–274
Murakami Y, Funada R, Sano Y, Ohtani J (1999) The differentiating of contact cells and isolation cells in the xylem ray parenchyma of Populus maximowiczii. Ann Bot 84:429–435
Parameswaran N, Liese W (1982) Ultrastructural localization of wall components in wood cells. Holz als Roh-und Werkstoff 40:145–155
Parameswaran N, Sinner M (1979) Topochemical studies on the wall of beech bark sclereids by enzymatic and acidic degradation. Protoplasma 101:197–215
Ruel K, Chevalier-Billosta V, Guillemin F, Sierra JB, Joseleau J-P (2006) The wood cell wall at the ultrastructural scale-formation and topochemical organization. Maderas Ciencia y Tecnol 8:107–116
Sannigrahi P, Ragauskas AJ, Tuskan GA (2010) Poplar as a feedstock for biofuels: a review of compositional characteristics. Biofuels Bioprod Bioref 4:209–226
Taylor G (2002) Populus: arabidopsis for forestry. Do we need a model tree? Ann Bot 90:681–689
Terashima N, fukushima K, Tsuchiya S (1986) Heterogeneity in formation of lignin. VII. An autoradiographic study on the formation of guaiacyl and syringyl lignin in poplar. J Wood Chem Technol 6:495–504
Terashima N, Awano T, Takabe K, Yoshida M (2004) Formation of macromolecular lignin in ginko xylem cell walls as observed by field emission scanning electron microscopy. C R Biologie 327:903–910
Terashima N, Kitano K, Kojima M, Yoshida M, Yamamoto H, Westermark U (2009) Nanostructural assembly of cellulose, hemicellulose, and lignin in the middle layer of secondary wall of ginko tracheid. J Wood Sci 55:409–416
Tokoh C, Takabe K, Sugiyama J, Fujita M (2002a) Cellulose synthesized by Acetobacter xylinum in the presence of plant cell wall polysaccharides. Cellulose 9:65–74
Tokoh C, Takabe K, Sugiyama J, Fujita M (2002b) CP/MAS 13C NMR and electron diffraction study of bacterial cellulose structure affected by cell wall polysaccharides. Cellulose 9:351–360
Vian B, Reis D, Mosiniak M, Roland JC (1986) The glucuronoxylans and the helicoidal shift in cellulose microfibrils in linden wood: cytochemistry in muro and on isolated molecules. Protoplasma 131:185–199
Vian B, Roland JC, Reis D, Mosiniak M (1992) Distribution and possible morphogenetic role of the xylans within the secondary vessel wall of linden wood. IAWA Bull n s 13:269–282
Willför S, Sundberg A, Pranovich A, Holmbom (2005) Polysaccharides in some industrially important hardwood species. Wood Sci Technol 39:601–617
Yoshinaga A, Fujita M, Saiki H (1993) Compositions of lignin building units and neutral sugars in oak xylem tissue. Mokuzai Gakkaishi 39:621–627
Acknowledgments
The authors gratefully acknowledge funding provided by the Formas FuncFiber Center of Excellence (http://www.funcfiber.se).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.S., Sandquist, D., Sundberg, B. et al. Spatial and temporal variability of xylan distribution in differentiating secondary xylem of hybrid aspen. Planta 235, 1315–1330 (2012). https://doi.org/10.1007/s00425-011-1576-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1576-8