Abstract
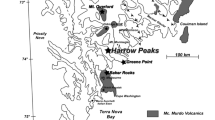
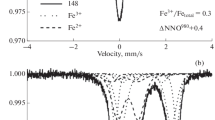
Amphibole is the hydrous metasomatic phase in spinel-bearing mantle xenoliths from Baker Rocks, Northern Victoria Land, Antarctica. It occurs in veins or in disseminated form in spinel lherzolites. Both types derive from reaction between metasomatic melts and the pristine paragenesis of the continental lithospheric mantle beneath Northern Victoria Land. To determine the effective role of water circulation during the metasomatic process and amphibole formation, six amphibole samples were fully characterized. Accurate determination of the site population and the state of dehydrogenation in each of these amphiboles was carried out using single-crystal X-ray diffraction, electron microprobe and secondary ion mass spectroscopy on the same single crystal. The Fe3+/ΣFe ratio was determined by X-ray absorption near edge spectroscopy on amphibole powder. The degree of dehydrogenation determined by SIMS is 0.870–0.994 O3(O2−) a.p.f.u., primary and ascribed to the Ti-oxy component of the amphibole, as indicated by atom site populations; post-crystallization H loss is negligible. Estimates of aH2O (0.014–0.054) were determined from the dehydration equilibrium among end-member components assuming that amphiboles are in equilibrium with the anhydrous peridotitic phases. A difference up to 58 % in determination of aH2O can be introduced if the chemical formula of the amphiboles is calculated based on 23 O a.p.f.u. without knowing the effective amount of dehydrogenation. The oxygen fugacity of the Baker Rocks amphibole-bearing mantle xenoliths calculated based upon the dissociation constant of water (by oxy-amphibole equilibrium) is between −2.52 and −1.32 log units below the fayalite–magnetite–quartz (FMQ) buffer. These results are systematically lower and in a narrow range of values relative to those obtained from anhydrous olivine–orthopyroxene–spinel equilibria (fO2 between −1.98 and −0.30 log units). A comparative evaluation of the two methods suggests that when amphibole is present in mantle peridotites, the application of oxy-amphibole equilibrium is preferred, because ol–opx–sp oxy-calibrations are not “sensitive” enough in recording the effects (if any) of amphibole in the peridotite matrix. Amphibole acts as the main H acceptor among the peridotite minerals and may prevent fluid circulation and buffer oxygen fugacity. The important conclusion of this study is that amphibole within the lithospheric mantle does not always means high water activity and oxidizing conditions.





Similar content being viewed by others
References
Annersten H, Olesch M, Seifert FA (1978) Ferric iron in orthopyroxene: a Mössbauer spectroscopic study. Lithos 11:301–310
Arai S (1992) Chemistry of chromian spinel in volcanic rocks as a potential guide to magma chemistry. Mineral Mag 56:173–184
Armienti P, Perinelli C (2010) Cenozoic thermal evolution of lithospheric mantle in northern Victoria Land (Antarctica): evidences from mantle xenoliths. Tectonophysics 486:28–35
Ballhaus C, Berr RF, Green DH (1991) High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen barometer: implications for the oxidation state of the upper mantle. Contrib Miner Petrol 108:384
Barnes SJ, Roeden PL (2001) The range of spinel compositions in terrestrial mafic and ultramafic rocks. J Petrol 42:2271–2302
Bonadiman C, Hao Y, Coltorti M, Dallai L, Faccini B, Huang Y, Xia Q (2009) Water contents of pyroxenes in intraplate lithospheric mantle. Eur J Mineral 21:637–647
Bosi F, Andreozzi GB, Ferrini V, Lucchesi S (2004) Behavior of cation vacancy in kenotetrahedral Cr-spinels from Albanian eastern belt ophiolites. Am Mineral 89:1367–1373
Brey GP, Köhler TP (1990) Geothermometry in four-phase lherzolites II: new thermobarometers and practical assessment of existing thermobarometers. J Petrol 31:1353–1378
Brown ID, Shannon RD (1973) Empirical bond strength-bond length curves for oxides. Acta Crystallogr A29:266–282
Canil D, O’Neill HSTC (1996) Distribution of Ferric iron in some upper-mantle assemblages. J Petrol 37:609–635
Canil D, O’Neill HSTC, Pearson DG, Rudnick RL, McDonough WF, Carswell DA (1994) Ferric iron in mantle peridotites and mantle oxidation states. Earth Planet Sci Lett 123:205–220
Carswell DA, Gibbs FGF (1987) Evaluation of mineral thermometers and barometers applicable to garnet lherzolite assemblages. Contrib Miner Petrol 95:499–511
Chakraborty S (1997) Rates and mechanism of Fe–Mg interdiffusion in olivine at 980–1300 °C. J Geophys Res 102:12317–12331
Chattarjee ND (1991) Applied mineralogical thermodynamics. Springer, Heidelberg
Chazot G, Menzies M, Harte B (1996) Silicate glasses in spinel lherzolites from Yemen: origin and chemical composition. Chem Geol 134:159–179
Coltorti M, Beccaluva L, Bonadiman C, Salvini L, Siena F (2000) Glasses in mantle xenoliths as geochemical indicators of metasomatic agents. Earth Planet Sci Lett 183:303–320
Coltorti M, Beccaluva L, Bonadiman C, Faccini B, Ntaflos T, Siena F (2004) Amphibole genesis via metasomatic reaction with clinopyroxene in mantle xenoliths from Victoria Land, Antarctica. Lithos 75:115–139
Coltorti M, Bonadiman C, Faccini B, Gregoire M, O’Reilly SY, Powell W (2007) Amphiboles from suprasubduction and intraplate lithospheric mantle. Lithos 99:68–84
Comodi P, Boffa-Ballaran T, Zanazzi PF, Capalbo C, Zanetti A, Nazzareni S (2010) The effect of oxo-component on the high-pressure behavior of amphiboles. Am Mineral 95:1042–1051
Dale J, Powell R, White RW, Elmer FL, Holland TJB (2005) A thermodynamic model for Ca–Na clinoamphiboles in Na2O–CaO–FeO–MgO–Al2O3–SiO2–H2O–O for petrological calculations. J Metamorph Geol 23:771–791
Dawson JB, Smith JV (1982) Upper mantle amphiboles: a review. Mineral Mag 45:35–46
Demény A, Harangi SZ, Vennemannc TW, Casillasd R, Horvátha P, Miltone AJ, Masonf PRD, Ulianovc A (2012) Amphiboles as indicators of mantle source contamination: combined evaluation of stable H and O isotope compositions and trace element ratios. Lithos 152:141–156
Dolejs D (2005) Evidence for fluoride melts in Earth’s mantle formed by liquid immiscibility: Comment and Reply: COMMENT. Geology 33:e76–e77
Dyar MD, McGuire AV, Ziegler RD (1989) Redox equilibria and crystal chemistry of coexisting minerals from spinel lherzolite mantle xenoliths. Am Mineral 74:969–980
Dyar MD, McGuire AV, Harrell MD (1992) Crystal chemistry of iron in two styles of metasomatism in the upper mantle. Geochim Cosmochim Acta 56:2579–2586
Dyar MD, Mackwell SM, McGuire AV, Cross LR, Robertson JD (1993) Crystal chemistry of Fe3+ and H+ in mantle kaersutite: implications for mantle metasomatism. Am Mineral 78:968–979
Dyar MD, Gunter ME, Delany JS, Lanzarotti AA, Sutton SR (2002) Systematics in the structure and XANES spectra of pyroxenes, amphiboles, and micas as derived from oriented single crystals. Can Mineral 40:1375–1393
Faccini B, Bonadiman C, Coltorti M, Grégoire M, Siena F (2013) Oceanic material recycled within the subpatagonian lithospheric mantle (Cerro del Fraile, Argentina). J Petrol 54:1211–1258
Farges F (2001) Crystal-chemistry of Fe in natural grandidierites: a XAFS spectroscopy study at the Fe K-edge. Phys Chem Miner 28:619–629
Foley SF (2011) A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. J Petrol 52:1363–1391
Foley SF, Taylor WR, Green DH (1986) The effect of fluorine on phase relationships in the system KAlSiO4–Mg2SiO4–SiO2 at 28 kbar and the solution mechanism of fluorine in silicate melts. Contrib Mineral Petrol 93:46–55
Frost DJ, McCammon CA (2008) The redox state of Earth’s mantle. Annual Rev Earth Planet Sci Lett 36:389–420
Ganguly J, Saxena S (1987) Mixtures and mineral reactions. Springer, Berlin
Gasparik T, Newton NC (1984) The reversed alumina contents of orthopyroxene in equilibrium with spinel and forsterite in the system MgO–Al2O3–SiO2. Contrib Mineral Petrol 85:186–196
Giuli G, Pratesi G, Paris E, Cipriani C (2002) Fe local structure in tektites and impactites by EXAFS and high-resolution XANES spectroscopy. Geochim Cosmochim Acta 66:4347–4353
Giuli G, Paris E, Hess KU, Dingwell DB, Cicconi MR, Eckhout SG, Fehr KT, Valenti P (2011) XAS determination of the Fe local environment and oxidation state in phonolite glasses and implications for the viscosity of silicate melts. Am Mineral 96:631–636
Goncharov AG, Ionov DA (2012) Redox state of deep off-craton lithospheric mantle: new data from garnet and spinel peridotites from Vitim, southern Siberia. Contrib Mineral Petrol 164:731–745
Hawthorne FC, Oberti R (2007) Amphiboles: crystal chemistry. In: Hawthorne FC, Oberti R, Della Ventura G, Mottana A (eds) Amphiboles: crystal chemistry, occurrence, and health issues. Reviews in Mineralogy and Geochemistry, vol 67, pp 1–51
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16:309–343
Holland TJB, Powell R (2006) Mineral activity-composition relations and petrological calculations involving cation equipartition in multisite minerals: a logical inconsistency. J Metamorph Geol 24:851–886
Ibers JA, Hamilton WC (1974) International tables for X-ray crystallography, vol 4. Kynoch Press, Birmingham
Klemme S (2004) Evidence for fluoride melts in Earth’s mantle formed by liquid immiscibility. Geology 32:441–444
Lamb WM, Popp RK (2009) Amphibole equilibria in mantle rocks: determining values of mantle aH2O and implications for mantle H2O contents. Am Mineral 94:41–52
Li J, Zhang S (2002) Redox state of amphibole-bearing mantle peridotite from Nüshan, Anhui Province in eastern China and its implications. Sci China (Series D) 45:348–357
Li ZXA, Lee CTA, Peslier AH, Lenardic A (2008) Water contents in mantle xenoliths from the Colorado Plateau and vicinity: Implications for the mantle rheology and hydration-induced thinning of continental lithosphere. J Geophys Res B09210:1–22
Liermann HP, Ganguly J (2003) Fe2+–Mg fractionation between orthopyroxene and spinel: experimental calibration in the system FeO–MgO–Al2O3–Cr2O3–SiO2 and applications. Contrib Mineral Petrol 145:217–227
Luth RW, Canil D (1993) Ferric iron in mantle-derived pyroxenes and a new oxybarometer for the mantle. Contrib Min Petrol 113:236–248
Mattioli GS, Wood B (1988) Magnetite activities across the MgAl2O4–Fe3O4 spinel join, with application to thermobarometric estimates of upper mantle oxygen fugacity. Contrib Mineral Petrol 98:148–162
McCammon C, Kopylova MG (2004) A redox profile of the Slave mantle and oxygen fugacity control in the cratonic mantle. Contrib Mineral Petrol 148:55–68
Melchiorre M, Coltorti M, Bonadiman C, Faccini B, O'Reilly SY, Pearson NJ (2011) The role of eclogite in the rift-related metasomatism and Cenozoic magmatism of Northern Victoria Land, Antarctica. Lithos 124:319–330
McGuire AV, Dyar MD, Nielson JE (1991) Metasomatic oxidation of upper mantle peridotite. Contrib Min Petrol 109:252–264
Mercier JC, Nicolas A (1975) Textures and fabrics of upper mantle peridotites as illustrated by xenoliths from basalts. J Petrol 16:454–487
Monkawa A, Mikouchi T, Koizumi E, Chokai J, Sugiyama K, Miyamoto M (2004) Iron micro-XANES analysis of martian kaersutites. Met Planet Sci 39:A71
Monkawa A, Mikouchi T, Koizumi E, Sugiyama K, Miyamoto M (2006) Determination of the Fe oxidation state of the Chassigny kaersutite: a microXANES spectroscopic study. Met Planet Sci 41:1321–1329
Morimoto N, Fabries J, Ferguson AK, Ginzburg IV, Ross M, Seifert FA, Zussman J, Aoki K, Gottardi G (1988) Nomenclature of pyroxenes. Mineral Mag 52:535–550
Mottana A, Paris E, Ventura GD, Robert JL (1990) Spectroscopic evidence for tetrahearally coordinated titanium in richteritic amphiboles. Rendiconti Lincei 1:387–392
Nazzareni S, Skogby H, Zanazzi PF (2010) Hydrogen content in clinopyroxene phenocrysts from salina mafic lavas (Aeolian arc, Italy). Contrib Mineral Petrol 162:275–288
Nell J, Wood BJ (1991) High-temperature electrical measurements and thermodynamic properties of Fe3O4–FeCr2O4–MgCr2O4–FeAl2O4 spinels. Am Mineral 76:405–426
O’Neill HSC, Wall VJ (1987) The olivine-orthopyroxene-spinel oxygen geobarometer, the nickel precipitation curve, and the oxygen fugacity of the Earths upper mantle. J Petrol 28:1169–1191
O’Reilly SY, Griffin WL (1988) Mantle metasomatism beneath western Victoria, Australia: I. Metasomatic processes in Cr-diopside lherzolites. Geochem Cosmochim Acta 52:433–447
Oberti R, Ungaretti L, Cannillo E, Hawthorne FC (1993) The mechanism of Cl incorporation in amphibole. Am Mineral 78:746–775
Oberti R, Vannucci R, Zanetti A, Tiepolo M, Brumm RC (2000) A crystal-chemical re-evaluation of amphibole/melt and amphibole/clinopyroxene DTi in petrogenetic studies. Am Mineral 85:407–419
Oberti R, Hawthorne FC, Cannillo E, Ca′mara F (2007) Long-range order in amphiboles. In: Hawthorne FC, Oberti R, Della Ventura G, Mottana A (eds) Amphiboles: crystal chemistry, occurrence, and health issues. Reviews in Mineralogy and Geochemistry, vol 67, pp 125–171
Ota K, Mikouchi T, Sugiyama K (2009) Crystallography of hornblende amphibole in LAP04840 R chondrite and implication for its metamorphic history. J Mineral Petrol Sci 104:215–225
Ottolini L, Bottazzi P, Vannucci R (1993) Quantification of lithium, beryllium and boron in silicates by secondary ion mass spectrometry using conventional energy filtering. Anal Chem 65:1960–1968
Ottolini L, Bottazzi P, Zanetti A, Vannucci R (1995) Determination of hydrogen in silicates by secondary ion mass spectrometry. The Analyst 120:1309–1314
Ottolini L, Cámara F, Hawthorne FC (2001) Quantification of H, B, F in kornerupine: Accuracy of SIMS and SREF (X-ray single-crystal structure refinement) data. Microchim Acta 20:1–5
Ottolini L, Cámara F, Hawthorne FC, Stirling J (2002) SIMS matrix effects in the analysis of light elements in silicate minerals: Comparison with SREF and EMPA data. Am Mineral 87:1477–1485
Parkinson IJ, Arculus RJ (1999) Redox state of subduction zones: insights from arc peridotites. Chem Geol 160:409–423
Pearson DG, Canil D, Shirey SB (2003) Mantle samples included in volcanic rocks: xenoliths and diamonds. In: Carlson RW (ed) Treatise on geochemistry, vol 2., The mantle and coreElsevier, Amsterdam, pp 171–276
Perinelli C, Armienti P, Dallai L (2006) Geochemical and O-isotope constraints on the evolution of lithospheric mantle in the Ross Sea rift area (Antarctica). Contrib Mineral Petrol 151:245–266
Perinelli C, Andreozzi GB, Conte AM, Oberti R, Armienti P (2012) Redox state of subcontinental lithospheric mantle and relationships with metasomatism: insights from spinel peridotites from northern Victoria Land (Antarctica). Contrib Mineral Petrol 164:1053–1067
Peslier AH (2010) A review of water contents of nominally anhydrous natural minerals in the mantles of Earth, Mars and the Moon. J Volcanol Geotherm Res 197:239–258
Peslier AH, Luhr JF, Post J (2002) Low water contents on pyroxenes from spinel peridotite of the oxidized, subarc mantle wedge. Earth Planet Sci Lett 201:69–86
Pike NJE, Schwartzman EC (1977) Classification textures in ultramafic xenoliths. J Geol 85:49–61
Popp RK, Hibbert HA, Lamb WM (2006) Oxy-amphibole equilibria in Ti-bearing calcic amphiboles: experimental investigation and petrologic implications for mantle-derived amphiboles. Am Mineral 91:54–66
Pouchou JL, Pichoir F (1984) Possibilités d’analyse en profondeur à la microsonde électronique. J Microsc Spectrosc Electron 9:99–100
Quintiliani M, Andreozzi GB, Graziani G (2006) Fe2+ and Fe3+ quantification by different approaches and fO2 estimation for Albanian Cr-spinels. Am Mineral 91:907–916
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Sobolev VN, McCammon CA, Taylor LA, Snyder GA, Sobolev NV (1999) Precise Mössbauer milliprobe determination of ferric iron in rock-forming minerals and limitations of electron microprobe analysis. Am Mineral 84:78–85
Tiepolo M, Zanetti A, Oberti R (1999) Detection, crystal-chemical mechanism and petrological implications of [6]Ti4+ partitioning in pargasite and kaersutite. Eur J Min 11:345–354
Tiepolo M, Vannucci R, Bottazzi, Oberti R, Zanetti A, Foley SF. (2000) Partitioning of REE, Y, Th, U and Pb between pargasite, kaersutite and basanite to trachyte melts: implications for percolated and veined mantle. Geochemistry, Geophysics, Geosystems, paper number 2000GC000064
Verma MPA (2003) Thermodynamic assessment of dissociation constant of water. In: Proceedings 28th workshop on geothermal reservoir engineering. Stanford University, Stanford, California, January 27–29, 2003 SGP-TR-173
Wang J, Hattori KH, Kilian R, Stern CR (2007) Metasomatism of sub-arc mantle peridotites below southernmost South America: reduction of fO2 by slab-melt. Contrib Mineral Petrol 153:607–624
Welch M, Shuangxi L, Klinowski J (1998) 29Si MAS NMR systematic of calcic and sodic–calcic amphiboles. Am Mineral 83:85–96
White RV, Powell R, Clark GL (2002) The interpretation of reaction texture in Fe-rich metapelitic granulites in Musgrave Bloc, Central Australia: constraints from mineral equilibria calculations in the system K2O–FeO–MgO–Al2O3–SiO2–H2O–TiO2–Fe2O3. J Metamorph Geol 20:41–55
Wilke M, Farges F, Petit PE, Brown GE, Martin F (2001) Oxidation state and coordination of Fe in minerals: an Fe K-XANES spectroscopic study. Am Mineral 86:714–730
Witt-Eickschen G, Harte B (1994) Distribution of trace-elements between amphibole and clinopyroxene from mantle peridotites of the Eifel (western Germany): an ion-microprobe study. Chem Geol 117:235–250
Wood BJ (1990) An experimental test of the spinel peridotite oxygen barometer. J Geophys Res 97:15845–15851
Wood BJ, Virgo D (1989) Upper mantle oxidation state: ferric iron contents of lherzolite spinels by 57Fe Mössbauer spectroscopy and resultant oxygen fugacities. Geoch Cosmochim Acta 53:1227–1291
Woodland AB, Kornprobst J, Tabit A (2006) Ferric iron in orogenic lherzolite massifs and controls of oxygen fugacity in the upper mantle. Lithos 89:222–241
Zanetti A, Mazzucchelli M, Rivalenti G, Vannucci R (1999) The Finero phlogopite peridotite massif: an example of subduction related metasomatism. Contrib Mineral Petrol 134:107–122
Zipfel J, Worner G (1992) Thermobarometry on four- and five-phase peridotites from a continental rift system: evidence for upper mantle uplift and cooling at the Ross Sea margin (Antarctica). Contrib Mineral Petrol 111:24–36
Acknowledgments
The manuscript benefited dramatically from the constructive comments of Dante Canil and an Anonymous Referee. Mark Welch is thanked for critical comments and suggestions. The authors would like to acknowledge Luisa Ottolini at IGG-CNR of Pavia and Raul Carampin at IGG-CNR of Padua for performing SIMS and EMP analyses. European Synchrotron Radiation Facility is acknowledged for allocating beamtime. This study was supported by the PNRA (Programma Nazionale Ricerche in Antartide) Project. 2010/A2.08 “Xenoliths and basic lavas in understanding the C–O–H system in the mantle of the polar regions”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. L. Grove.
Rights and permissions
About this article
Cite this article
Bonadiman, C., Nazzareni, S., Coltorti, M. et al. Crystal chemistry of amphiboles: implications for oxygen fugacity and water activity in lithospheric mantle beneath Victoria Land, Antarctica. Contrib Mineral Petrol 167, 984 (2014). https://doi.org/10.1007/s00410-014-0984-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-014-0984-8