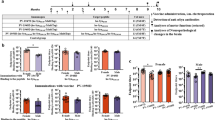
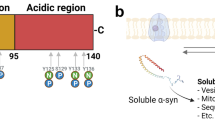
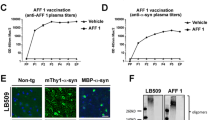
Abstract
Immunotherapeutic approaches are currently in the spotlight for their potential as disease-modifying treatments for neurodegenerative disorders. The discovery that α-synuclein (α-syn) can transmit from cell to cell in a prion-like fashion suggests that immunization might be a viable option for the treatment of synucleinopathies. This possibility has been bolstered by the development of next-generation active vaccination technology with short peptides-AFFITOPEs® (AFF)- that do not elicit an α-syn-specific T cell response. This approach allows for the production of long term, sustained, more specific, non-cross reacting antibodies suitable for the treatment of synucleinopathies, such as Parkinson’s disease (PD). In this context, we screened a large library of peptides that mimic the C-terminus region of α-syn and discovered a novel set of AFF that identified α-syn oligomers. Next, the peptide that elicited the most specific response against α-syn (AFF 1) was selected for immunizing two different transgenic (tg) mouse models of PD and Dementia with Lewy bodies, the PDGF- and the mThy1-α-syn tg mice. Vaccination with AFF 1 resulted in high antibody titers in CSF and plasma, which crossed into the CNS and recognized α-syn aggregates. Active vaccination with AFF 1 resulted in decreased accumulation of α-syn oligomers in axons and synapses, accompanied by reduced degeneration of TH fibers in the caudo-putamen nucleus and by improvements in motor and memory deficits in both in vivo models. Clearance of α-syn involved activation of microglia and increased anti-inflammatory cytokine expression, further supporting the efficacy of this novel active vaccination approach for synucleinopathies.











Similar content being viewed by others
References
Amor S, Puentes F, Baker D, van der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129(2):154–169. doi:10.1111/j.1365-2567.2009.03225.x
Amschl D, Neddens J, Havas D, Flunkert S, Rabl R, Romer H, Rockenstein E, Masliah E, Windisch M, Hutter-Paier B (2013) Time course and progression of wild type alpha-synuclein accumulation in a transgenic mouse model. BMC Neurosci 14:6. doi:10.1186/1471-2202-14-6
Angot E, Brundin P (2009) Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 3):S143–S147. doi:10.1016/S1353-8020(09)70802-8
Aquilano K, Baldelli S, Rotilio G, Ciriolo MR (2008) Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem Res 33(12):2416–2426. doi:10.1007/s11064-008-9697-6
Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM (2007) Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 27(34):9115–9129. doi:10.1523/JNEUROSCI.2361-07.2007
Bach P, Tschäpe JA, Kopietz F, Braun G, Baade JK, Wiederhold KH, Staufenbiel M, Prinz M, Deller T, Kalinke U, Buchholz CJ, Müller UC (2009) Vaccination with Abeta-displaying virus-like particles reduces soluble and insoluble cerebral Abeta and lowers plaque burden in APP transgenic mice. J Immunol 182(12):7613–7624. doi:10.4049/jimmunol.0803366
Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, Desplats P, Masliah E, Lee SJ (2012) Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci 32(39):13454–13469. doi:10.1523/JNEUROSCI.1292-12.2012
Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, Schettini G (1999) Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J Neurochem 73(6):2348–2357
Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ (1997) A new class of membrane-bound chemokine with a CX3C motif. Nature 385(6617):640–644. doi:10.1038/385640a0
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924. doi:10.1038/nn1715
Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR (2012) A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics: J Am Soc Exp Neurotherap 9(2):297–314. doi:10.1007/s13311-012-0104-2
Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT Jr (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA 97(2):571–576
Cook A, Hippensteel R, Shimizu S, Nicolai J, Fatatis A, Meucci O (2010) Interactions between chemokines: regulation of fractalkine/CX3CL1 homeostasis by SDF/CXCL12 in cortical neurons. J Biol Chem 285(14):10563–10571. doi:10.1074/jbc.M109.035477
Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E (2010) Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One 5(2):e9313. doi:10.1371/journal.pone.0009313
Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M (2007) Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci 27(34):9220–9232. doi:10.1523/JNEUROSCI.2617-07.2007
de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73(4):685–697. doi:10.1016/j.neuron.2011.11.033
Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 106(31):13010–13015. doi:10.1073/pnas.0903691106
Eizenberg O, Faber-Elman A, Lotan M, Schwartz M (1995) Interleukin-2 transcripts in human and rodent brains: possible expression by astrocytes. J Neurochem 64(5):1928–1936
Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF (2004) Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci 24(42):9434–9440. doi:10.1523/JNEUROSCI.3080-04.2004
Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47. doi:10.1186/1750-1326-4-47
Games D, Seubert P, Rockenstein E, Patrick C, Trejo M, Ubhi K, Ettle B, Ghassemiam M, Barbour R, Schenk D, Nuber S, Masliah E (2013) Axonopathy in an alpha-synuclein transgenic model of Lewy body disease is associated with extensive accumulation of C-terminal-truncated alpha-synuclein. Am J Pathol 182(3):940–953. doi:10.1016/j.ajpath.2012.11.018
Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O (2012) alpha-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis 47(2):258–267. doi:10.1016/j.nbd.2012.04.009
Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L (1998) Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA 95(18):10896–10901
Hashimoto M, Hsu LJ, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E (1999) Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport 10(4):717–721
Hashimoto M, Masliah E (1999) Alpha-synuclein in Lewy body disease and Alzheimer’s disease. Brain Pathol 9(4):707–720
Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH (2002) Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia 37(4):314–327
Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T (1995) The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14(2):467–475
Iwatsubo T, Yamaguchi H, Fujimuro M, Yokosawa H, Ihara Y, Trojanowski JQ, Lee VM (1996) Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am J Pathol 148(5):1517–1529
Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI (2012) Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem 287(23):19440–19451. doi:10.1074/jbc.M112.346072
Kohler G, Milstein C (1976) Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol 6(7):511–519. doi:10.1002/eji.1830060713
Kramer ML, Schulz-Schaeffer WJ (2007) Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci 27(6):1405–1410. doi:10.1523/JNEUROSCI.4564-06.2007
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318
Lalonde R, Qian S (2007) Exploratory activity, motor coordination, and spatial learning in Mchr1 knockout mice. Behav Brain Res 178(2):293–304. doi:10.1016/j.bbr.2007.01.006
Lalonde R, Strazielle C (2009) Exploratory activity and motor coordination in old versus middle-aged C57BL/6J mice. Arch Gerontol Geriatr 49(1):39–42. doi:10.1016/j.archger.2008.04.009
Lansbury PT Jr (1999) Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci USA 96(7):3342–3344
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14(1):38–48. doi:10.1038/nrn3406
Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT (2002) Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol 322(5):1089–1102
Lee HJ, Suk JE, Bae EJ, Lee SJ (2008) Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun 372(3):423–428. doi:10.1016/j.bbrc.2008.05.045
Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ (2010) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285(12):9262–9272. doi:10.1074/jbc.M109.081125
Lee SJ, Lim HS, Masliah E, Lee HJ (2011) Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci Res 70(4):339–348. doi:10.1016/j.neures.2011.05.008
Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7(2):e31302. doi:10.1371/journal.pone.0031302
Luthi-Carter R (2003) Progress towards a vaccine for Huntington’s disease. Mol Ther 7(5 Pt 1):569–570
Mandler M, Rockenstein E, Ubhi K, Hansen L, Adame A, Michael S, Galasko D, Santic R, Mattner F, Masliah E (2012) Detection of peri-synaptic amyloid-β pyroglutamate aggregates in early stages of Alzheimer’s disease and in AβPP transgenic mice using a novel monoclonal antibody. J Alzheimers Dis 28(4):783–794. doi:10.3233/JAD-2011-111208
Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D (2005) Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 46(6):857–868. doi:10.1016/j.neuron.2005.05.010
Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D (2011) Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One 6(4):e19338. doi:10.1371/journal.pone.0019338
Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287(5456):1265–1269
Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L (2001) beta-Amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA 98(21):12245–12250. doi:10.1073/pnas.211412398
McKeith IG (2000) Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurol Clin 18(4):865–902
Menéndez-González M, Pérez-Piñera P, Martínez-Rivera M, Muñiz AL, Vega JA (2011) Immunotherapy for Alzheimer’s disease: rational basis in ongoing clinical trials. Curr Pharm Des 17(5):508–520
Metz GA, Schwab ME (2004) Behavioral characterization in a comprehensive mouse test battery reveals motor and sensory impairments in growth-associated protein-43 null mutant mice. Neuroscience 129(3):563–574. doi:10.1016/j.neuroscience.2004.07.053
Miller TW, Shirley TL, Wolfgang WJ, Kang X, Messer A (2003) DNA vaccination against mutant huntingtin ameliorates the HDR6/2 diabetic phenotype. Mol Ther 7(5 Pt 1):572–579
Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW (2000) A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature 408(6815):982–985. doi:10.1038/35050116
Murphy DD, Rueter SM, Trojanowski JQ, Lee VM (2000) Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci 20(9):3214–3220
Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M (1998) Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett 429(2):167–172
Okuno T, Nakatsuji Y, Kumanogoh A, Moriya M, Ichinose H, Sumi H, Fujimura H, Kikutani H, Sakoda S (2005) Loss of dopaminergic neurons by the induction of inducible nitric oxide synthase and cyclooxygenase-2 via CD 40: relevance to Parkinson’s disease. J Neurosci Res 81(6):874–882. doi:10.1002/jnr.20599
Olanow CW, Prusiner SB (2009) Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci USA 106(31):12571–12572. doi:10.1073/pnas.0906759106
Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D (1997) Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 387(6633):611–617. doi:10.1038/42491
Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL (2007) Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem 282(8):5862–5870. doi:10.1074/jbc.M608126200
Ransohoff RM (2009) Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31(5):711–721. doi:10.1016/j.immuni.2009.09.010
Rockenstein E, Crews L, Masliah E (2007) Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv Drug Deliv Rev 59(11):1093–1102. doi:10.1016/j.addr.2007.08.013
Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E (2002) Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res 68(5):568–578. doi:10.1002/jnr.10231
Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2013) Incidence and pathology of synucleinopathies and tauopathies related to Parkinsonism. JAMA Neurol :1–7. doi:10.1001/jamaneurol.2013.114
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400(6740):173–177. doi:10.1038/22124
Schneeberger A, Mandler M, Mattner F, Schmidt W (2010) AFFITOME® technology in neurodegenerative diseases: the doubling advantage. Hum Vaccin 6(11):948–952
Schneeberger A, Mandler M, Mattner F, Schmidt W (2012) Vaccination for Parkinson’s disease. Parkinsonism Relat Disord 18(Suppl 1):S11–S13. doi:10.1016/S1353-8020(11)70006-2
Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S (2010) A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci 30(24):8083–8095. doi:10.1523/JNEUROSCI.1091-10.2010
Sigurdsson EM, Brown DR, Daniels M, Kascsak RJ, Kascsak R, Carp R, Meeker HC, Frangione B, Wisniewski T (2002) Immunization delays the onset of prion disease in mice. Am J Pathol 161(1):13–17. doi:10.1016/S0002-9440(10)64151-X
Singh VK, Mehrotra S, Agarwal SS (1999) The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res 20(2):147–161
Solomon B (2007) Active immunization against Alzheimer’s beta-amyloid peptide using phage display technology. Vaccine 25(16):3053–3056. doi:10.1016/j.vaccine.2007.01.069
Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A (2005) Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res 1040(1–2):202–207. doi:10.1016/j.brainres.2005.01.100
Spencer B, Michael S, Shen J, Kosberg K, Rockenstein E, Patrick C, Adame A, Masliah E (2012) Lentivirus mediated delivery of neurosin promotes clearance of wild-type α-synuclein and reduces the pathology in an α-synuclein model of LBD. Mol Therapy: J Am Soc Gene Therapy. doi:10.1038/mt.2012.66
Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E (2009) Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci 29(43):13578–13588. doi:10.1523/JNEUROSCI.4390-09.2009
Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflamm 1(1):14. doi:10.1186/1742-2094-1-14
Trojanowski JQ, Lee VM (1998) Aggregation of neurofilament and alpha-synuclein proteins in Lewy bodies: implications for the pathogenesis of Parkinson disease and Lewy body dementia. Arch Neurol 55(2):151–152
Troquier L, Caillierez R, Burnouf S, Fernandez-Gomez FJ, Grosjean ME, Zommer N, Sergeant N, Schraen-Maschke S, Blum D, Buee L (2012) Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res 9(4):397–405
Tsigelny IF, Bar-On P, Sharikov Y, Crews L, Hashimoto M, Miller MA, Keller SH, Platoshyn O, Yuan JX, Masliah E (2007) Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. FEBS J 274(7):1862–1877. doi:10.1111/j.1742-4658.2007.05733.x
Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Spencer B, Rockenstein E, May V, Winkler J, Masliah E (2012) Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of α-synucleinopathy. Exp Neurol 234(2):405–416. doi:10.1016/j.expneurol.2012.01.008
Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, Whitney K, Masliah E (2010) Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci 30(18):6236–6246. doi:10.1523/JNEUROSCI.0567-10.2010
Valera E, Masliah E (2013) Immunotherapy for neurodegenerative diseases: focus on α-synucleinopathies. Pharmacol Ther. doi:10.1016/j.pharmthera.2013.01.013
van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G (2000) Neuropathology in mice expressing human alpha-synuclein. J Neurosci 20(16):6021–6029
Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr (1996) NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 35(43):13709–13715. doi:10.1021/bi961799n
Wilcock DM, Colton CA (2008) Anti-amyloid-beta immunotherapy in Alzheimer’s disease: relevance of transgenic mouse studies to clinical trials. J Alzheimers Dis 15(4):555–569
Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R (2011) In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA 108(10):4194–4199. doi:10.1073/pnas.1100976108
Acknowledgments
We thank Andrea Achleitner, Martina-Anna Gschirtz, Michael Hierzer, Beate Pilz, Martina Trefil and Christina Wöss for their contribution in conducting the experiments. This work was funded by the National Institutes of Health (NIH) grants NS044233, AG18440, NS047303, AG022074 and NS057096. In addition, funding was provided by Austrian Science promotion agency (FFG) grants 813335, 817969, 821453 and by the Michael J. Fox foundation for Parkinson's research (MJFF) grant: AFFITOPE® based immunotherapeutic strategies for Parkinson's disease.
Conflict of interest
The authors Markus Mandler, Harald Weninger, Radmila Santic, Stefanie Meindl, Benjamin Vigl, Oskar Smrzka and Achim Schneeberger are employees of AFFiRiS, the company that commercializes the AFFITOPEs® described in the manuscript. The author Frank Mattner is co-founder of AFFiRiS. The authors Elvira Valera, Edward Rockenstein, Christina Patrick, Anthony Adame and Eliezer Masliah declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Mandler and E. Valera are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mandler, M., Valera, E., Rockenstein, E. et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson’s disease clinical trials. Acta Neuropathol 127, 861–879 (2014). https://doi.org/10.1007/s00401-014-1256-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1256-4