Abstract
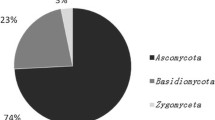
Cryoconite holes have biogeochemical, ecological and biotechnological importance. This communication presents results on culturable psychrophilic yeast and filamentous fungi from cryoconite holes at Midre Lovénbreen glacier. The identification of these microbes was achieved through conventional and DNA sequencing techniques. Effect of temperature, salt and media on growth of the cultures was studied. Measurements on the bioavailability of nutrients and trace metals were made through different methods including ICPMS (Inductively Coupled Plasma Mass Spectrometry). Colony forming unit (CFU) per gram of sediment sample was calculated to be about 7 × 103–1.4 × 104 and 4 × 103–1.2 × 104 of yeast and filamentous fungi, respectively. Based on morphology and sequence data, these were identified as Cryptococcus gilvescens, Mrakia sp., Rhodotorula sp., Phialophora alba and Articulospora tetracladia. Amongst these, Phialophora alba, Cryptococcus gilvescens and Mrakia sp. zhenx-1 are reported for the first time from Svalbard Arctic, while Rhodotorula sp. (95% gene similarity) is a new species, yet to be described. Rhodotorula sp. expressed high amylase, while Cryptococcus gilvescens showed high lipase activity. Mrakia sp. showed phosphate solubilization between 4 and 15°C, which is a first record. Chemical analysis revealed the presence of organic carbon, nitrogen and phosphorus in substantial amounts in the sediments. Filamentous fungi and yeast in the cryoconite holes drive the process of organic macromolecule degradation through cold-adapted enzyme secretion, thereby assisting in nutrient cycling in these subglacial environments. Further, these cold-adapted enzymes may provide an opportunity for the prospect of biotechnology in Arctic. This is the first report on mycological investigation into cryoconite holes from Midre Lovénbreen glacier.





Similar content being viewed by others
References
Anesio AM, Mindl B, Laybourn-Parry J, Hodson AJ, Sattler B (2007) Viral dynamics in cryoconite holes on a high Arctic glacier (Svalbard). J Geophys Res doi:10.1029/2006JG000350
Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B (2009) High microbial activity on glaciers: importance to the global carbon cycle. Global Change Biol 15:955–960
Barnett HL (1960) Illustrated genera of imperfect fungi, 2nd edn. Burgess Publishing Company, USA
Barron GL (1977) The genera of hyphomycetes from soil. Robert E. Krieger Pub. Comp, INC. Huntington, New York
Bray RH, Kurtz LT (1945) Determination of total organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Butinar L, Spencer-Martins I, Gunde-Cimerman N (2007) Yeasts in high Arctic glaciers: the discovery of a new habitat for eukaryotic microorganisms. Antonie Leeuwenhoek 91:277–289
Buzzini P, Martini A (2002) Extracellular enzymatic activity profiles in yeast and yeast-like strains isolated from tropical environments. J Appl Microbiol 93:1020–1025
Carmichael JW, BryceKendrick W, Conners IL, Sigler L (1980) Genera of hyphomycetes. The University of Alberta Press, Canada
Christner BC, Kvitko BH, Reeve JN (2003) Molecular identification of bacteria and eukarya inhabiting an Antarctic cryoconite hole. Extremophiles 7:177–183
Edwards A, Anesio AM, Rassner SM, Sattler B, Hubbard B, Perkins WT, Young M, Griffith GW (2011) Possible interactions between bacterial diversity, microbial activity and supraglacial hydrology of cryoconite holes in Svalbard. ISME J 5:150–160
Ellis MB (1971) Dematiaceous hyphomycetes. CMI, Kew, England
Ellis MB (1976) More dematiaceous hyphomycetes. CMI, Kew, England
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A (2000) Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Micr 50:1351–1371
Fiedurek J, Gromada A, Saomka A, Korniaowicz-Kowalska T, Kurek E, Melke J (2003) Catalase activity in Arctic microfungi grown at different temperatures. Acta Biol Hung 54:105–112
Foght J, Aislabie J, Turner S, Brown CE, Ryburn J, Saul DJ, Lawson W (2004) Culturable bacteria in subglacial sediments and ice from two southern hemisphere glaciers. Microb Ecol 47:329–340
Gostinĉar C, Urŝiĉ V, De Hoog S, Gunde-Cimerman N (2006) Local evolution of black yeast A. pullulans in sub glacial Arctic ice. In: Proceedings of international conference on alpine and polar microbiology, Innsbruck, Austria, p 19
Hagen JO, Kohler J, Melvold K, Winther JG (2003) Glaciers in Svalbard: mass balance, runoff and freshwater flux. Polar Res 22:145–159
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron 4:1–9
Hankin L, Anagnostakis SL (1975) The use of solid media for detraction of enzyme production by fungi. Mycologia 67:97–607
Hodson A, Anesio AM, Ng F, Watson R, Quirk J Irvine-Fynn T et al. (2007) A glacier respires: quantifying the distribution and respiration CO2 flux of cryoconite across an entire Arctic supraglacial ecosystem. J Geophys Res doi:10.1029/2007JG000452
Hodson AJ, Anesio AM, Tranter M, Fountain AG, Osborn M, Priscu J, Laybourn-parry J, Sattler B (2008) Glacial ecosystems. Ecol Monogr 78:41–67
Kastovska K, Elster J, Stibal M, Santruckova H (2005) Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (High Arctic). Microb Ecol 50:396–407
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Ainsworth and Bisby’s dictionary of the fungi, 10th edn. CABI Publishing, UK
Libkind D, Brizzio S, Ruffini A, Gadanho M, Van Broock M, Paulo SJ (2003) Molecular characterization of carotenogenic yeasts from aquatic environments in Patagonia, Argentina. Antonie Leeuwenhoek 84:313–322
Mindl B, Anesio AM, Meirer K, Hodson AJ, Laybourn-Parry J, Sommaruga R, Sattler B (2007) Factors influencing bacterial dynamics along a transect from supralgacial runoff to proglacial lakes of a high Arctic glacier. FEMS Microbiol Ecol 59:307–317
Mueller DR, Vincent WF, Pollard WH, Fristen CH (2001) Glacial cryconite ecosystems: a bipolar comparison of algal communities and habitats. Nova Hedwigia 123:173–197
Säwström C, Mumford P, Marshall W, Hodson A, Laybourn-parry J (2002) The microbial communities and primary productivity of cryoconite holes in Arctic glacier (Svalbard 79N). Polar Biol 25:591–596
Säwström C, Grane′li W, Laybourn-Parry J, Anesio AM (2007) High viral infection rates in Antarctic and Arctic bacterioplankton. Environ Microbiol 9:250–255
Sharp M, Parkes J, Cragg B, Fairchild IJ, Lamb H, Tranter M (1999) Widespread bacterial populations at glacier beds and their relationships to rock weathering and carbon cycling. Geology 27:107–110
Skidmore ML, Foght JM, Sharp MJ (2000) Microbial life beneath a High Arctic glacier. Appl Environ Microbiol 66:3214–3220
Skidmore ML, Anderson SP, Sharp MJ, Foght JM, Lanoil BD (2005) Comparison of microbial community composition of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Appl Environ Microb 71:6986–6997
Skowronek M, Kuszewska J, Fiedurek J, Gromada A (2003) Invertase activity of psychrotrophic fungi. Annales Universitatis Mariae Curie-Skłodowska Lublin-Polonia 58:1–9
Stibal M, Anesio AM, Blues CJD, Tranter M (2009) Phosphatase activity and organic phosphorus turnover on a high Arctic glacier. Biogeosciences 6:913–922
Stonehouse B (1989) Polar ecology. Chapman and Hall, New York
Subbiah BH, Asija GL (1956) A rapid procedure for determination of available nitrogen in soils. Curr Sci 25:259–260
Takeuchi N, Kohshima S, Seko K (2001) Structure, formation, and darkening process of albedo-reducing material (cryoconite) on a Himalayan glacier: a granular algal mat growing on the glacier. Arct Antarct Alp Res 33:115–122
Turchetti B, Buzzini P, Goretti M, Branda E, Diolaiuti G, D’Agata C, Smiraglia C, Vaughan-Martini A (2008) Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol Ecol 63:73–83
Waksman SA (1916) Do fungi live and produce mycelium in the soil? Science NS 44:320–322
Walkley A, Black CA (1934) An examination of Degtjareff methods for determining soil organic matter and a proposed modification of chromic acid titration method. Soil Sci 37:29–38
Wharton RA, McKay CP, Simmons GM, Parker BC (1985) Cryoconite holes on glaciers. Bioscience 35:499–503
Yarrow D (1998) Methods for the isolation, maintenance and identification of yeasts. The Yeasts. In: Kurtzman CP, Fell JW (eds) A taxonomic study. Elsevier, Amsterdam, pp 77–100
Acknowledgments
Author (PS1) is highly indebted to Department of Science & Technology (DST), New Delhi for financial support. Authors are thankful to Dr Rasik Ravindra, Director NCAOR, Director BITS and Dr Jagdev Sharma, NRCG for encouragement and facilities. Thanks are due to Dr C. T. Achuthankutty for improving the English language of the manuscript, Chief Editor and anonymous reviewers for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, P., Singh, S.M. Characterization of yeast and filamentous fungi isolated from cryoconite holes of Svalbard, Arctic. Polar Biol 35, 575–583 (2012). https://doi.org/10.1007/s00300-011-1103-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1103-1