Abstract
Purpose
To evaluate the per-patient diagnostic performance of a minimized non-contrast MRI protocol for hepatocellular carcinoma (HCC) surveillance in cirrhotic liver, as well as factors affecting diagnostic sensitivity.
Methods
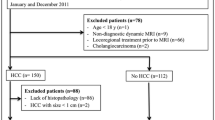
A total of 226 patients who underwent MRI for HCC surveillance over an 8 year period were included in this retrospective study. Set1 consisted of diffusion-weighted imaging and respiratory-triggered, fast-spin echo T2-weighted imaging with fat suppression. Set2 included T1-weighted in/opposed-phase images added to the images from Set1. Image sets were scored as positive or negative for HCC according to predetermined criteria by two readers independently. The diagnostic performance of the two sets in conjunction with α-fetoprotein (AFP) was assessed and compared using the McNemar test. Logistic regression was used to determine factors that affected sensitivity.
Results
The sensitivity, specificity, and accuracy of Set1 of readers 1 and 2 were 84.4%/87.3%, 86.8%/86.8%, and 85.0%/87.2%, respectively; and those for Set2 were 87.3%/89.6%, 81.1%/79.2%, and 85.8/87.2%, respectively. The sensitivities of the sets were not significantly different (p = 0.063). Sensitivities of both sets in conjunction with AFP were higher than those of MRI alone without statistical significance (87.3%/89.6%, p = 0.063/> 0.99; 89.6%/89.6%, p = 0.125/> 0.99). In very early-stage HCC, the sensitivities of Sets1 and 2 were 73.1%/76.9% and 76.9%/82.7%, respectively. Perihepatic ascites and size less than 2 cm were associated with sensitivity (p < 0.05).
Conclusions
A minimized non-contrast MRI protocol consisting of Fat-sat T2WI and DWI is highly sensitive and may be a viable method for HCC surveillance of the cirrhotic liver. The inclusion of T1-weighted in/opposed-phase and AFP may increase this sensitivity.





Similar content being viewed by others
References
B. Stewart, C.P. Wild, World cancer report 2014, Self (2018).
B.-H. Zhang, B.-H. Yang, Z.-Y. Tang, Randomized controlled trial of screening for hepatocellular carcinoma, Journal of cancer research and clinical oncology 130(7) (2004) 417-422.
T. Sato, R. Tateishi, H. Yoshida, T. Ohki, R. Masuzaki, J. Imamura, T. Goto, F. Kanai, S. Obi, N. Kato, S. Shiina, T. Kawabe, M. Omata, Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C, Hepatology international 3(4) (2009) 544-50.
J.A. Marrero, L.M. Kulik, C. Sirlin, A.X. Zhu, R.S. Finn, M.M. Abecassis, L.R. Roberts, J.K. Heimbach, Diagnosis, staging and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases, Hepatology (2018).
P.R. Galle, A. Forner, J.M. Llovet, V. Mazzaferro, F. Piscaglia, J.-L. Raoul, P. Schirmacher, V. Vilgrain, EASL clinical practice guidelines: management of hepatocellular carcinoma, Journal of hepatology (2018).
K.N. National Cancer Center, K.L.C.S. Group, 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma, Gut and liver 9(3) (2015) 267.
N. Kokudo, K. Hasegawa, M. Akahane, H. Igaki, N. Izumi, T. Ichida, S. Uemoto, S. Kaneko, S. Kawasaki, Y. Ku, Evidence‐based C linical P ractice G uidelines for H epatocellular C arcinoma: The J apan S ociety of H epatology 2013 update (3rd JSH‐HCC G uidelines), Hepatology Research 45(2) (2015).
C. Verslype, O. Rosmorduc, P. Rougier, E.G.W. Group, Hepatocellular carcinoma: ESMO–ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of oncology 23(suppl_7) (2012) vii41-vii48.
A. Colli, M. Fraquelli, G. Casazza, S. Massironi, A. Colucci, D. Conte, P. Duca, Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review, The American journal of gastroenterology 101(3) (2006) 513.
S.Y. Kim, J. An, Y.-S. Lim, S. Han, J.-Y. Lee, J.H. Byun, H.J. Won, S.J. Lee, H.C. Lee, Y.S. Lee, MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma, JAMA oncology 3(4) (2017) 456-463.
K. Tzartzeva, J. Obi, N.E. Rich, N.D. Parikh, J.A. Marrero, A. Yopp, A.K. Waljee, A.G. Singal, Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis, Gastroenterology 154(6) (2018) 1706-1718. e1.
Y.K. Kim, Y.K. Kim, H.J. Park, M.J. Park, W.J. Lee, D. Choi, Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma, Magnetic resonance imaging 32(6) (2014) 610-618.
S. Han, J.-I. Choi, M.Y. Park, M.H. Choi, S.E. Rha, Y.J. Lee, The Diagnostic Performance of Liver MRI without Intravenous Contrast for Detecting Hepatocellular Carcinoma: A Case-Controlled Feasibility Study, Korean Journal of Radiology 19(4) (2018) 568-577.
M. Kim, T.W. Kang, D.I. Cha, K.M. Jang, Y.K. Kim, S.H. Kim, D.H. Sinn, K. Kim, Identification of Arterial Hyperenhancement in CT and MRI in Patients with Hepatocellular Carcinoma: Value of Unenhanced Images, Korean J Radiol 20(2) (2019) 236-245.
EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma, J Hepatol 56(4) (2012) 908-43.
J.R. Landis, G.G. Koch, The measurement of observer agreement for categorical data, biometrics (1977) 159-174.
R.M. Marks, A. Ryan, E.R. Heba, A. Tang, T.J. Wolfson, A.C. Gamst, C.B. Sirlin, M.R. Bashir, Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance, AJR. American journal of roentgenology 204(3) (2015) 527-35.
S. Han, J.I. Choi, M.Y. Park, M.H. Choi, S.E. Rha, Y.J. Lee, The Diagnostic Performance of Liver MRI without Intravenous Contrast for Detecting Hepatocellular Carcinoma: A Case-Controlled Feasibility Study, Korean J Radiol 19(4) (2018) 568-577.
M. Di Martino, D. Marin, A. Guerrisi, M. Baski, F. Galati, M. Rossi, S. Brozzetti, R. Masciangelo, R. Passariello, C. Catalano, Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis, Radiology 256(3) (2010) 806-16.
N.C. Yu, V. Chaudhari, S.S. Raman, C. Lassman, M.J. Tong, R.W. Busuttil, D.S. Lu, CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis, Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 9(2) (2011) 161-7.
J.K. Heimbach, L.M. Kulik, R.S. Finn, C.B. Sirlin, M.M. Abecassis, L.R. Roberts, A.X. Zhu, M.H. Murad, J.A. Marrero, AASLD guidelines for the treatment of hepatocellular carcinoma, Hepatology 67(1) (2018) 358-380.
D.G. Mitchell, J. Bruix, M. Sherman, C.B. Sirlin, LI‐RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI‐RADS Management Working Group and future directions, Hepatology 61(3) (2015) 1056-1065.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J.S., Lee, J.K., Baek, S.Y. et al. Diagnostic performance of a minimized protocol of non-contrast MRI for hepatocellular carcinoma surveillance. Abdom Radiol 45, 211–219 (2020). https://doi.org/10.1007/s00261-019-02277-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02277-9