Abstract
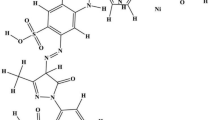
Geobacter metallireducens was found to be capable of decolorizing several azo dyes with different structures to various extents. Pyruvate, ethanol, acetate, propionate, and benzoate could support 66.3 ± 2.6−93.7 ± 2.1 % decolorization of 0.1 mM acid red 27 (AR27) in 40 h. The dependence of the specific decolorization rate on AR27 concentration (25 to 800 μM) followed Michaelis–Menten kinetics (K m = 186.9 ± 1.4 μΜ, V max = 0.65 ± 0.02 μmol mg protein−1 h−1). Enhanced AR27 decolorization was observed with the increase of cell concentrations ranging from 7.5 to 45 mgL−1. AR27 decolorization by G. metallireducens was retarded by the presence of goethite, which competed electrons with AR27 and was reduced to Fe(II). The addition of low concentrations of humic acid (1−100 mgL−1) or 2-hydroxy–1,4-naphthoquinone (0.5−50 μM) could improve the decolorization performance of G. metallireducens. High-performance liquid chromatography analysis suggested reductive pathway to be responsible for decolorization. This was the first study on azo dye decolorization by Geobacter strain and might improve our understanding of natural attenuation and bioremediation of environments polluted by azo dyes.





Similar content being viewed by others
References
Anderson RT, Vrionis HA, Ortiz-Bernad I, Resch CT, Peacock A, Dayvault R, Marutzky S, Metzler DR, Karp K, Lowe M, White DC, Long PE, Lovley DR (2003) Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl Environ Microbiol 69:5884–5891
Bazylinski DA, Dean AJ, Schuler D, Phillips EJP, Lovley DR (2000) N2-dependent growth and nitrogenase activity in the metal-metabolizing bacteria, Geobacter and Magnetospirillum species. Environ Microbiol 2:266–273
Bond DR, Holmes DE, Tender LM, Lovley DR (2002) Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483–485
Boukhalfa H, Icopini GA, Reilly SD, Neu MP (2007) Plutonium (IV) reduction by the meal-reducing bacteria Geobacter metallireducens G15 and Shewanella oneidensis MR1. Appl Environ Microbiol 73:5897–5903
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brigé A, Motte B, Borloo J, Buysschaert G, Devreese B, van Beeumen JJ (2008) Bacterial decolorization of textile dyes is an extracellular process requiring a multicomponent electron transfer pathway. Microbiol Biotechnol 1:40–52
Brown MA, DeVito SC (1993) Predicting azo dye toxicity. Crit Rev Environ Sci Technol 23:249–324
Cai P, Xiao X, He Y, Li W, Chu J, Wu C, He M, Zhang Z, Sheng G, Lam MHW, Xu F, Yu H (2011) Anaerobic biodecolorization mechanism of methyl orange by Shewanella oneidensis MR-1. Appl Microbiol Biotechnol. doi:10.1007/s00253-011-3508-8
Cervantes FJ, Vu-Thi-Thu L, Lettinga G, Field JA (2004) Quinone-respiration improves dechlorination of carbon tetrachloride by anaerobic sludge. Appl Microbiol Biotechnol 64:702–711
Chen X, Sun G, Xu M (2010) Role of iron in azoreduction by resting cells of Shewanella decolorationis S12. J Appl Microbiol 110:580–586
Coates JD, Ellis DJ, Blunt-Harris EL, Gaw CV, Roden EE, Lovley DR (1998) Recovery of humic-reducing bacteria from a diversity of environments. Appl Environ Microbiol 64:1504–1509
Dos Santos AB, Cervantes FJ, Van Lier JB (2007) Review paper on current technologies for decolourization of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour Technol 98:2369–2385
Dubrow SF, Boardman GD, Michelsen DL (1996) Chemical pretreatment and aerobic–anaerobic degradation of textile dye wastewater. In: Reife A and Freeman HS (eds) Environmental chemistry of dyes and pigments. Wiley Interscience, San Diego, CA. pp 75–102
Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JLM, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM (2008) Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603
Hong Y, Guo J, Xu Z, Xu M, Sun G (2007a) Humic substances act as electron acceptor and redox mediator for microbial dissimilatory azoreduction by Shewanella decolorationis S12. J Microbiol Biotechnol 17:428–437
Hong Y, Xu M, Guo J, Xu Z, Chen X, Sun G (2007b) Respiration and growth of Shewanella decolorationis S12 with an azo compound as sole electron acceptor. Appl Environ Microbiol 73:64–72
Hori T, Muller A, Igarashi Y, Conrad R, Friedrich MW (2010) Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. ISME J 4:267–278
Jahn MK, Haderlein SB, Meckenstock RU (2006) Reduction of Prussian Blue by the two iron-reducing microorganisms Geobacter metallireducens and Shewanella alga. Environ Microbiol 8:362–367
Khalid A, Arshad M, Crowley DE (2008) Decolorization of azo dyes by Shewanella sp. under saline conditions. Appl Microbiol Biotechnol 79:1053–1059
Kwon MJ, Finneran KT (2006) Microbially mediated biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine by extracellular electron shuttling compounds. Appl Environ Microbiol 72:5933–5941
Kwon MJ, Finneran KT (2008a) Biotransformation products and mineralization potential for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in abiotic versus biological degradation pathways with anthraquinone-2,6-disulfonate (AQDS) and Geobacter metallireducens. Biodegradation 19:705–715
Kwon MJ, Finneran KT (2008b) Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) biodegradation kinetics amongst several Fe(III)-reducing bacteria. Soil Sed Contam 17:189–203
Lin B, Westerhoff HV, Röling WFM (2009) How Geobacteraceae may dominate subsurface biodegradation: physiology of Geobacter metallireducens in slow-growth habitat-stimulating retentostats. Environ Microbiol 11:2425–2433
Liu G, Wang J, Lu H, Jin R, Zhou J, Zhang L (2009a) Effects of reduction products of ortho-hydroxyl substituted azo dyes on biodecolorization of azo dyes. J Hazard Mater 171:222–229
Liu G, Zhou J, Meng X, Fu QS, Wang J, Jin R, Lv H (2012) Decolorization of azo dyes by marine Shewanella strains under saline conditions. Appl Microbiol Biotechnol. doi:10.1007/s00253-012-4216-8
Liu G, Zhou J, Wang J, Wang X, Jin R, Lv H (2011) Decolorization of azo dyes by Shewanella oneidensis MR-1 in the presence of humic acids. Appl Microbiol Biotechnol 91:417–424
Liu G, Zhou J, Wang J, Zhou M, Lu H, Jin R (2009b) Acceleration of azo dye decolorization by using quinone reductase activity of azoreductase and quinone redox mediator. Bioresour Technol 100:2791–2795
Lloyd JR, Macaskie LE (1996) A novel PhosphorImager-based technique for monitoring the microbial reduction of technetium. Appl Environ Microbiol 62:578–582
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382:445–448
Lovley DR, Fraga JL, Blunt-Harris EL, Hayes LA, Phillips EJP, Coates JD (1998) Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol 26:152–157
Lovley DR, Giovannoni SJ, White DC, Champine JE, Phillips EJP, Gorby YA, Goodwin S (1993) Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol 159:336–344
Lovley DR, Philips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Lovley DR, Phillips EJP, Gorby YA, Landa ER (1991) Microbial reduction of uranium. Nature 350:413–416
Lovley DR, Ueki T, Zhang T, Malvankar NS, Shrestha PM, Flanagan KA, Aklujkar M, Butler JE, Giloteaux L, Rotaru AE, Holmes DE, Franks AE, Orellana R, Risso C, Nevin KP (2011) Geobacter: the microbe electric’s physiology, ecology, and practical applications. Adv Microb Physiol 59:1–100
McCormick ML, Adriaens P (2004) Carbon tetrachloride transformation on the surface of nanoscale biogenic magnetite particles. Environ Sci Technol 38:1045–1053
McCormick ML, Bouwer EJ, Adriaens P (2002) Carbon tetrachloride transformation in a model iron-reducing culture: relative kinetics of biotic and abiotic reactions. Environ Sci Technol 36:403–410
Meng X, Liu G, Zhou J, Fu QS, Wang G (2012) Azo dye decolorization by Shewanella aquimarina under saline conditions. Bioresour Technol 114:95–101
Ortiz-Bernad I, Anderson RT, Vrionis HA, Lovley DR (2004) Vanadium respiration by Geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl Environ Microbiol 70:3091–3095
Pearce CI, Christie R, Boothman C, von Canstein H, Guthrie JT, Lloyd JR (2006) Reactive azo dye reduction by Shewanella strain J18 143. Biotechnol Bioeng 95:692–703
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigm 58:179–196
Rau J, Knackmus HJ, Stolz A (2002) Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ Sci Technol 36:1497–1504
Roden EE, Scheibe TD (2005) Conceptual and numerical model of uranium(VI) reductive immobilization in fractured subsurface sediments. Chemosphere 59:617–628
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Engrs 42:138–157
Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol 56:69–80
Tremblay PL, Aklujkar M, Leang C, Nevin KP, Lovley DR (2011) A genetic system for Geobacter metallireducens: role of the flagellin and pilin in the reduction of Fe(III) oxide. Environ Microbiol Rep 4:82–88
Van der Zee FP, Cervantes FJ (2009) Impact and application of electron shuttles on the redox (bio)transformation of contaminants: a review. Biotechnol Adv 27:256–277
Van Trump JI, Sun Y, Coates JD (2006) Microbial interactions with humic substances. Adv Appl Microbiol 60:55–96
Wang X, Cheng X, Sun D (2008) Autocatalysis in reactive black 5 biodecolorization by Rhodopseudomonas palustris W1. Appl Microbiol Biotechnol 80:907–915
Wolf M, Kappler A, Jiang J, Meckenstock RU (2009) Effects of humic substances and quinones at low concentrations on ferrihydrite reduction by Geobacter metallireducens. Environ Sci Technol 43:5679–5685
Xu H, Heinze TM, Chen S, Cerniglia CE, Chen H (2007) Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes (Sudan dyes) by human intestinal microflora. Appl Environ Microbiol 73:7759–7762
Yang YY, Du LN, Wang G, Jia XM, Zhao YH (2011) The decolorisation and mechanism of Shewanella oneidensis MR-1 for methyl orange and acid yellow 199 under microaerophilic conditions. Water Sci Technol 63:956–963
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (No. 51008044, 50978040), Fundamental Research Funds for the Central Universities, and China Postdoctoral Science Foundation (201104596).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 208 kb)
Rights and permissions
About this article
Cite this article
Liu, G., Zhou, J., Chen, C. et al. Decolorization of azo dyes by Geobacter metallireducens . Appl Microbiol Biotechnol 97, 7935–7942 (2013). https://doi.org/10.1007/s00253-012-4545-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4545-7