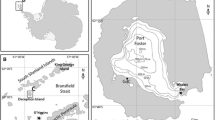
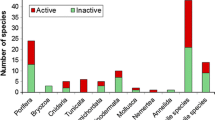
Abstract
Hexactinellids (glass sponges) are an understudied class with syncytial organization and poor procariotic associations, thought to lack defensive secondary metabolites. Poriferans, though, are outstanding sources of bioactive compounds; nonetheless, a growing suspicion suggests that many of these chemicals could be symbiont-derived. In Polar latitudes, sponges are readily invaded by diatoms, which could provide natural products. Hexactinellids are typical of deep waters; but in Antarctica, they dominate the upper shelf providing shelter and food supply to many opportunistic mesograzers and macroinvertebrates, which exert strong ecological pressures on them. Aiming to examine the incidence of defensive activities of hexactinellids against consumption, feeding experiments were conducted using their lipophilic fractions. Antarctic hexactinellid and demosponge extracts were tested against the asteroid Odontaster validus and the amphipod Cheirimedon femoratus as putative sympatric, omnivorous consumers. Hexactinellids yielded greater unpalatable activities towards the amphipod, while no apparent allocation of lipophilic defenses was noted. After chemical analyses on the lipophilic fractions from these Antarctic glass sponges, quite similar profiles were revealed, and no peculiar secondary metabolites, comparable to those characterizing other poriferans, were found. Instead, the lipidic compounds 5α(H)-cholestan-3-one and two glycoceramides were isolated for their particular outspread presence in our samples. The isolated compounds were further assessed in asteroid feeding assays, and their occurrence was evaluated for chemotaxonomical purposes in all the Antarctic samples as well as in glass sponges from other latitudes by NMR and MS. Characteristic sphingolipids are proposed as chemical markers in Hexactinellida, with possible contributions to the classification of this unsettled class.







Similar content being viewed by others
References
Abbas S, Sims J, Kelly M, Bowling J, Hamman M (2011) Advancement into the Arctic region for bioactive secondary metabolites. Mar Drugs 9:2423–2437
Amsler CD, Moeller CB, McClintock JB, Iken KB, Baker BJ (2000) Chemical defenses against diatom fouling in Antarctic marine sponges. Biofouling 16:29–45
Amsler MO, McClintock JB, Amsler CD, Angus RA, Baker BJ (2009) An evaluation of sponge-associated amphipods from the Antarctic Peninsula. Antarc Sci 21(6):579–589
Aumack CF, Amsler CD, McClintock JB, Baker BJ (2010) Chemically mediated resistance to mesoherbivory in finely branched macroalgae along the western Antarctic Peninsula. Eur J Phycol 45(1):19–26
Avila C, Iken K, Fontana A, Gimino G (2000) Chemical ecology of the Antarctic nudibranch Bathydoris hodgsoni Eliot, 1907: defensive role and origin of its natural products. J Exp Mar Biol Ecol 252:27–44
Avila C, Taboada S, Núñez-Pons L (2008) Marine Antarctic chemical ecology: what is next? Mar Ecol 29:1–70
Ayling A (1983) Growth and regeneration rates in thinly encrusting Desmospongiae from temperate waters. Biol Bull 165:343–352
Barthel D (1992) Antarctic hexactinellids: a taxonomically difficult, but ecologically important benthic component. Verh Dtsch Zool Ges 85(2):271–276
Barthel D (1995) Tissue composition of sponges from the Weddell Sea, Antarctica—not much meat on the bones. Mar Ecol Prog Ser 123(1–3):149–153
Barthel D, Gutt J (1992) Sponge associations in the eastern Weddell Sea. Antarc Sci 4:137–150
Barthel D, Tendal OS (1994) Antarctic Hexactinellida. In: Wägele JW, Sieg J (eds) Synopses of Antarctic benthos. Koeltz Scientific Books, Champaing, Illinois, pp 9–135
Bavestrello G, Arillo A, Calcinai B, Cattaneo-Vietti R, Cerrano C, Gaino E, Penna A, Sara M (2000) Parasitic diatoms inside Antarctic sponges. Biol Bull 198(1):29–33
Bergquist PR, Hofheinz W, Oesterhelt G (1980) Sterol composition and the classification of the Demospongiae. Biochem SystEcol 8(4):423–435
Bergquist PR, Lavis A, Cambie RC (1986) Sterol composition and classification of the Porifera. Biochem Syst Ecol 14(1):105–112
Bergquist PR, Karuso P, Cambie RC, Smith DJ (1991) Sterol composition and classification of the Porifera. Biochem Syst Ecol 19(1):17–24
Blumenberg M, Thiel V, Pape T, Michaelis W (2002) The steroids of hexactinellid sponges. Naturwissenschaften 89(9):415–419
Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR (2011) Marine natural products. Nat Prod Rep 28:196–268
Bobzin SC, Faulkner DJ (1992) Chemistry and chemical ecology of the Bahamian sponge Aplysilla glacialis. J Chem Ecol 18:309–332
Bregazzi PK (1972) Life cycles and seasonal movements of Cheirimedon femoratus and Tryphosella kergueleni Crustacea Amphipoda. Brit Antarct Surv B 30:1–34
Breitmaier E, Voelter W (1989) 13C NMR spectra of natural products. In: Ebel HF (ed) Carbon-13 NMR spectroscopy. VCH, New York, pp 340–358
Brusca RC, Brusca GJ (2003) Invertebrates. McGraw-Hill Interamericana en España, SAU, Madrid
Cattaneo-Vietti R, Bavestrello G, Cerrano C, Sarà A, Benatti U, Giovine M, Gaino E (1996) Optical fibres in an Antarctic sponge. Nature 383:397–398
Cerrano C, Arillo A, Bavestrello G, Calcinai B, Cattaneo-Vietti R, Penna A, Sara M, Totti C (2000) Diatom invasion in the Antarctic hexactinellid sponge Scolymastra joubini. Polar Biol 23(6):441–444
Cerrano C, Calcinai B, Cucchiari E, Di Camillo C, Nigro M, Regoli F, Sara A, Schiaparelli S, Totti C, Bavestrello G (2004a) Are diatoms a food source for Antarctic sponges? Chem Ecol 20:S57–S64
Cerrano C, Calcinai B, Cucchiari E, Di Camillo C, Totti C, Bavestrello G (2004b) The diversity of relationships between Antarctic sponges and diatoms: the case of Mycale acerata Kirkpatrick, 1907 (Porifera, Demospongiae). Polar Biol 27(4):231–237
Chanas B, Pawlik JR (1995) Defenses of Caribean sponges against predatory reef fish. II. Spicules, tissue toughness, and nutritional quality. Mar Ecol Prog Ser 127:195–211
Cruz-Rivera E, Hay ME (2003) Prey nutritional quality interacts with chemical defenses to affect consumer feeding and fitness. Ecol Monogr 73(3):483–506
Dayton PK (1979) Observations of growth, dispersal and population dynamics of some sponges in McMurdo Sound, Antarctica. In: Levi N and Bourny-Esnault (eds) Sponge Biology. Centre de recherché Scientifique, Paris, pp. 271–282
Dayton PK, Robillia GA, Paine RT, Dayton LB (1974) Biological accommodation in benthic community at McMurdo Sound Antarctica. Ecol Monog 44(1):105–128
De Broyer C, Lowry JK, Jazdzewski k, Robert H (2007) Catalogue of the Gammaridean and Corophiidean Amphipoda of the Southern Ocean, with distribution and ecological data. In: De Broyer C (ed) Census of Antarctic Marine Life: Synopsis of the Amphipoda of the Southern Ocean. Bull Inst R Sc N B-B 77, supplement 1, pp. 1–325
Duffy JE, Paul VJ (1992) Prey nutritional quality and the effectiveness of chemical defenses against tropical reef fishes. Oecologia 90(3):333–339
Falsone G, Budzikiewicz H, Wendisch D (1987) Constituents of euphorbiaceae. 9. New cerebrosides from Euphorbia biglandulosa desf. Z Naturforsch B 42(11):1476–1480
Furrow FB, Amsler CD, McClintock JB, Baker BJ (2003) Surface sequestration of chemical feeding deterrents in the Antarctic sponge Latrunculia apicalis as an optimal defense against sea star spongivory. Mar Biol 143(3):443–449
Gaino E, Bavestrello G, Cattaneovietti R, Sara M (1994) Scanning electron-microscope evidence for diatom uptake by 2 Antarctic sponges. Polar Biol 14(1):55–58
Goad LJ (1981) Sterol biosynthesis and metabolism in marine-invertebrates. Pure Appl Chem 53(4):837–852
Göcken C, Janussen D (2011) ANT XXIV/2 (SYSTCO) Hexactinellida (Porifera) and bathymetric traits of Antarctic glass sponges (incorporating ANDEEP-material); including an emendation of the rediscovered genus Lonchiphora. Deep-Sea Res II
Guella G, Mancini I, Pietra F (1988) Isolation of ergosta-4, 24(28)-dien-3-one from both Astrophorida demosponges and sub-Antarctic hexactinellides. Comp Biochem Phys B Comp Biochem 90(1):113–115
Gutt J (2007) Antarctic macro-zoobenthic communities: a review and an ecological classification. Antartc Sci 19(2):165–182
Hagernann A, Voigt O, Worheide G, Thiel V (2008) The sterols of calcareous sponges (Calcarea, Porifera). Chem Phys Lipids 156(1–2):26–32
Hayakawa K, Handa N, Ikuta N, Fukuchi M (1996) Downward fluxes of fatty acids and hydrocarbons during a phytoplankton bloom in the Austral summer in Breid Bay, Antarctica. Org Geochem 24(5):511–521
Hentschel U, Usher KM, Taylor MW (2006) Marine sponges as microbial fermenters. FEMS Microbiol Ecol 55(2):167–177
Huang YM, Amsler MO, McClintock JB, Amsler CD, Baker BJ (2007) Patterns of gammaridean amphipod abundance and species composition associated with dominant subtidal macroalgae from the western Antarctic Peninsula. Polar Biol 30(11):1417–1430
Janussen D, Tendal OS (2007) Diversity and distribution of Porifera in the bathyal and abyssal Weddell Sea and adjacent areas. Deep-Sea Res Pt II 54(16–17):1864–1875
Jayatilake GS, Thornton MP, Leonard AC, Grimwade JE, Baker BJ (1996) Metabolites from an Antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J Nat Prod 59(3):293–296
Jones AC, Blum JE, Pawlik JR (2005) Testing for defensive synergy in Caribbean sponges: bad taste or glass spicules? J Exp Mar Biol Ecol 322(1):67–81
Kunzmann K (1996) Associated fauna of selected sponges (Hexactinellida and Demospongiae) from the Weddell Sea, Antarctica. Ber Polarforsch 210:1–93
Lawson MP, Bergquist PR, Cambie RC (1984) Fatty-acid composition and the classification of the Porifera. Biochem Syst Ecol 12(4):375–393
Lawson MP, Stoilov IL, Thompson JE, Djerassi C (1988) Sterols in marine invertebrates, 59. Cell-membrane localization of sterols with conventional and unusual side-chains in 2 marine demosponges. Lipids 23(8):750–754
Leong W, Pawlik JR (2010) Evidence of a resource trade-off between growth and chemical defenses among Caribbean coral reef sponges. Mar Ecol Prog Ser 406:71–78
Leys SP (2003) The significance of syncytial tissues for the position of the Hexactinellida in the metazoa. Integr Comp Biol 43(1):19–27
Leys SP, Lauzon NRJ (1998) Hexactinellid sponge ecology: growth rates and seasonality in deep water sponges. J Exp Mar Biol Ecol 230(1):111–129
Leys SP, Mackie GO, Meech RW (1999) Impulse conduction in a sponge. J Exp Biol 202(9):1139–1150
Leys SP, Mackie GO, Reiswig HM (2007) The biology of glass sponges. Adv Mar Biol 52:1–145
McClintock JB (1987) Investigation of the relationship between invertebrate predation and biochemical composition, energy content, spicule armament and toxicity of benthic sponges at McMurdo Sound, Antarctica. Mar Biol 94(3):479–487
McClintock JB (1994) Trophic biology of Antarctic echinoderms. Mar Ecol Prog Ser 111:191–202
McClintock JB, Baker BJ, Amsler CD, Barlow TL (2000) Chemotactic tube-foot responses of the spongivorous sea star Perknaster fuscus to organic extracts of sponges from McMurdo Sound, Antarctica. Antarc Sci 12(1):41–46
McClintock JB, Amsler CD, Baker BJ, Van Soest R (2005) Ecology of Antarctic marine sponges: an overview. Integr Comp Biol 45:359–368
McClintock JB, Amsler CD, Baker B (2010) Overview of the chemical ecology of benthic marine invertebrates along the western Antarctic Peninsula. Integr Comp Biol 50(6):967–980
Müller WEG, Wendt K, Geppert C, Wiens M, Reiber A, Schröder HC (2006) Novel photoreception system in sponges?: unique transmission properties of the stalk spicules from the hexactinellid Hyalonema sieboldi. Biosens Bioelectron 21(7):1149–1155
Muralidhar P, Radhika P, Krishna N, Venkata Rao D, Bheemasankara Rao C (2003) Sphingolipids from marine organisms: a review. Nat Prod Sci 9(9):117–142
Nyssen F (2005) Role of benthic amphipods in Antarctic trophodynamics: a multidisciplinary study. University of Liege, Dissertation
Olguín HF, Alder VA (2011) Species composition and biogeography of diatoms in Antarctic and sub-Antarctic (Argentine shelf) waters (37–761S). Deep-Sea Res II 58:139–152
Padrón JM (2006) Sphingolipids in anticancer therapy. Curr Med Chem 13:755–770
Paul VJ (1992) Ecological roles of marine natural products. Comstock Publications Association, Ithaca, New York
Peters KJ, Amsler CD, McClintock JB, van Soest RWM, Baker BJ (2009) Palatability and chemical defenses of sponges from the western Antarctic Peninsula. Mar Ecol Prog Ser 385:77–85
Reiswig HM, Mackie GO (1983) Studies on Hexactinellid sponges. 3. The taxonomic status of Hexactinellida within the Porifera. Philos T Roy Soc B 301(1107):419–428
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal GA and Janzen DH (eds) Herbivores: their interaction with secondary plant metabolites. Academic Press, Inc., New York, USA, pp. 3–54
Rhoades DF, Gates RG (1976) Toward a general theory of plant antiherbivore chemistry. Recent Adv Phytochem 10:168–213
Sabdono A, Radjasa OK (2008) Microbial symbionts in marine sponges: marine natural product factory. J Coast Dev 11(2):57–61
Santalova EA, Makarieva TN, Gorshkova IA, Dmitrenok AS, Krasokhin VB, Stonik VA (2004) Sterols from six marine sponges. Biochem Syst Ecol 32:153–167
Santalova EA, Makarieva TN, Ponomarenko LP, Denisenko VA, Krasokhin VB, Mollo E, Cimino G, Stonik VA (2007) Sterols and related metabolites from five species of sponges. Biochem Syst Ecol 35:439–446
Sarà M, Bavestrello G, Cattaneo-Vietti R, Cerrano C (1998) Endosymbiosis in sponges: relevance for epigenesis and evolution. Symbiosis 25(1–3):57–70
Seldes AM, Rovirosa J, SanMartin A, Gros EG (1986) Steroids from aquatic organisms, 12. Sterols from the antarctic sponge Homaxinella balfourensis (Ridley and Dendy). Comp Biochem Physiol B 83(4):841–842
Seldes A, Romero M, Gros E, Darias J, Rovirosa J, San Martin A (1990a) Esteroides de las esponjas antárticas Cinachyra barbata Sollas y Xestospongia sp. Serie Científica Instituto Antártico Chileno 40:81–97
Seldes AM, Deluca ME, Gros EG, Rovirosa J, SanMartin A, Darias J (1990b) Steroids from aquatic organisms. 19. New sterols from antarctic sponge Artemisina apollonis. Z Naturforsch B 45(1):83–86
Slattery M, Hamann MT, McClintock JB, Perry TL, Puglisi MP, Yoshida WY (1997) Ecological roles for water-borne metabolites from Antarctic soft corals. Mar Ecol Prog Ser 161:133–144
Smith AC, Goodfellow R, Goad LJ (1972) The intermediacy of 3-oxo steroids in the conversion of cholest-5-en-3β-ol into 5α-cholestan-3β-ol by the starfish Asterias rubens and Porania pulvillus. Biochem J 128:1371–1372
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of stadistics in biological research. Freeman, W. H. and Co.: New York, p 937
Sotka EE, Forbey J, Horn M, Poore AGB, Raubenheimer D, Whalen KE (2009) The emerging role of pharmacology in understanding consumer–prey interactions in marine and freshwater systems. Integr Comp Biol 49(3):291–313
Takeuchi M, Sakane T, Yanagi M, Yamasato K, Hamana K, Yokota A (1995) Taxonomic study of bacteria isolated from plants—proposal of Sphingomonas rosa sp-nov, Sphingomonas pruni sp-nov, Sphingomonas asaccharolytica sp-nov, and Sphingomonas mali sp-nov. Int J Syst Bacteriol 45(2):334–341
Tan RX, Chen JH (2003) The cerebrosides. Natural Product Reports 20(5):509–534. doi:10.1039/b307243f
Taylor GD, Smith SO, Gagosian RB (1981) Use of microbial enrichments for the study of the anaerobic degradation of cholesterol. Geochim Cosmochim Ac 45:2161–2168
Taylor MW, Hill RT, Piel J, Thacker RW, Hentschel U (2007a) Soaking it up: the complex lives of marine sponges and their microbial associates. ISME J 1(3):187–190
Taylor MW, Radax R, Steger D, Wagner M (2007b) Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71:295–347
Thiel V, Blumenberg M, Hefter J, Pape T, Pomponi S, Reed J, Reitner J, Worheide G, Michaelis W (2002) A chemical view of the most ancient metazoan—biomarker chemotaxonomy of hexactinellid sponges. Naturwissenschaften 89(2):60–66
Thoms C, Schupp PJ, Custodio MR, Lobo-Hajdu G, Hajdu E, Muricy G (2007) Chemical defense strategies in sponges: a review. Porifera research: biodiversity, innovation and sustainability [Serie Livros 28]: 627–637
Tomaschko KH (1994) Ecdysteroids from Pycnogonum litorale (Arthropoda, Pantopoda) act as chemical defense against Carcinus maenas (Crustacea, Decapoda). J Chem Ecol 20(7):1445–1455
Tompkins-MacDonald GJ, Leys SP (2008) Glass sponges arrest pumping in response to sediment: implications for the physiology of the hexactinellid conduction system. Mar Biol 154(6):973–984
Toth GB, Karlsson M, Pavia H (2007) Mesoherbivores reduce net growth and induce chemical resistance in natural seaweed populations. Oecologia 152(2):245–255
van Meer G, Hoetzl S (2010) Sphingolipid topology and the dynamic organization and function of membrane proteins. FEBS Lett 584(9):1800–1805
Waddell B, Pawlik JR (2000) Defenses of Caribbean sponges against invertebrate predators. II. Assays with sea stars. Mar Ecol Prog Ser 195:133–144
Walters KD, Pawlik JR (2005) Is there a trade-off between wound-healing and chemical defenses among Caribbean reef sponges? Integr Comp Biol 45(2):352–358
Wulff J (2010) Regeneration of sponges in ecological context: is regeneration an integral part of life history and morphological strategies? Integr Comp Biol 50(4):494–505
Acknowledgements
We thank J. Vázquez, S. Taboada, B. Figuerola, M. Paone and E. Manzo for their precious help in the lab. Thanks are due to D. Janussen, S. Leys, T. Pérez and M. Bergmann (AWI) for kindly supplying hexactinellid samples from Northern latitudes. Also, we are grateful to W. Arntz and the crew of R/V Polarstern. UTM (CSIC), R/V “Las Palmas” and BAE “Gabriel de Castilla” crews provided logistic support. The “Centres Científics i Tecnològics” of the UB also provided technical support. Funding was provided by the Ministry of Science and Innovation of Spain (CGL2004-03356/ANT, CGL2007-65453/ANT and CGL2010-17415/ANT) and REDES Project (CGL2009/06185-E).
Ethical standards
We declare that this research conforms to the legal requirements of the Spanish and Italian laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 56.5 kb)
Rights and permissions
About this article
Cite this article
Núñez-Pons, L., Carbone, M., Paris, D. et al. Chemo-ecological studies on hexactinellid sponges from the Southern Ocean. Naturwissenschaften 99, 353–368 (2012). https://doi.org/10.1007/s00114-012-0907-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-012-0907-3