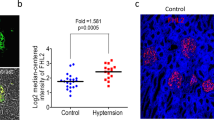
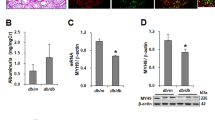
Abstract
Podocytes are significant in establishing the glomerular filtration barrier. Sustained rennin–angiotensin system (RAS) activation is crucial in the pathogenesis of podocyte injury and causes proteinuria. This study demonstrates that angiotensin II (Ang II) caused a reactive oxygen species (ROS)-dependent rearrangement of cortical F-actin and a migratory phenotype switch in cultured mouse podocytes with stable Ang II type 1 receptor (AT1R) expression. Activated small GTPase Rac-1 and phosphorylated ezrin/radixin/moesin (ERM) proteins provoked Ang II-induced F-actin cytoskeletal remodeling. This work also shows increased expression of Rac-1 and phosphorylated ERM proteins in cultured podocytes, and in glomeruli of podocyte-specific AT1R transgenic rats (Neph-hAT1 TGRs). The free radical scavenger DMTU eliminated Ang II-induced cell migration, ERM protein phosphorylation and cortical F-actin remodeling, indicating that ROS mediates the influence of Rac-1 on podocyte AT1R signaling. Heparin, a potent G-coupled protein kinase 2 inhibitor, was found to abolish ERM protein phosphorylation and cortical F-actin ring formation in Ang II-treated podocytes, indicating that phosphorylated ERM proteins are the cytoskeletal effector in AT1R signaling. Moreover, Ang II stimulation triggered down-regulation of α actinin-4 and reduced focal adhesion expression in podocytes. Signaling inhibitor assay of Ang II-treated podocytes reveals that Rac-1, RhoA, and F-actin reorganization were involved in expressional regulation of α actinin-4 in AT1R signaling. With persistent RAS activation, the Ang II-induced phenotype shifts from being dynamically stable to adaptively migratory, which may eventually exhaust podocytes with a high actin cytoskeletal turnover, causing podocyte depletion and focal segmental glomerulosclerosis.








Similar content being viewed by others
References
Remuzzi G, Benigni A, Remuzzi A (2006) Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116:288–296
Henger A, Huber T, Fischer KG, Nitschke R, Mundel P, Schollmeyer P, Greger R, Pavenstadt H (1997) Angiotensin II increases the cytosolic calcium activity in rat podocytes in culture. Kidney Int 52:687–693
Nitschke R, Henger A, Ricken S, Gloy J, Muller V, Greger R, Pavenstadt H (2000) Angiotensin II increases the intracellular calcium activity in podocytes of the intact glomerulus. Kidney Int 57:41–49
Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, Bleich M, Schollmeyer P, Greger R, Pavenstadt H (1997) Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J Clin Invest 99:2772–2781
Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83:253–307
Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, Koike H, Shimizu F, Kawachi H (2007) Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol 170:1841–1853
Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N (2004) Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15:1475–1487
Regitz-Zagrosek V, Neuss M, Holzmeister J, Warnecke C, Fleck E (1996) Molecular biology of angiotensin receptors and their role in human cardiovascular disease. J Mol Med 74:233–251
Tryggvason K, Pikkarainen T, Patrakka J (2006) Nck links nephrin to actin in kidney podocytes. Cell 125:221–224
Durvasula RV, Shankland SJ (2006) Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens 15:1–7
Smoyer WE, Mundel P (1998) Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med 76:172–183
Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, Bonomelli M, Mundel P, Endlich K, Remuzzi A, Remuzzi G (2006) Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol 168:1073–1085
Schiwek D, Endlich N, Holzman L, Holthofer H, Kriz W, Endlich K (2004) Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int 66:91–101
Bek MF, Bayer M, Muller B, Greiber S, Lang D, Schwab A, August C, Springer E, Rohrbach R, Huber TB, Benzing T, Pavenstadt H (2006) Expression and function of C/EBP homology protein (GADD153) in podocytes. Am J Pathol 168:20–32
Stachon A, Stegemann H, Hohage H, Rahn KH, Schlatter E (1998) Effects of diadenosine polyphosphates on the intracellular Ca2+ concentration in endothelial cells. Cell Physiol Biochem 8:175–184
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Ohara Y, Peterson TE, Harrison DG (1993) Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91:2546–2551
Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK (2002) Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91:406–413
Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK (2006) Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A 103:7432–7437
Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3:586–599
Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24:251–256
Dandapani SV, Sugimoto H, Matthews BD, Kolb RJ, Sinha S, Gerszten RE, Zhou J, Ingber DE, Kalluri R, Pollak MR (2007) Alpha-actinin-4 is required for normal podocyte adhesion. J Biol Chem 282:467–477
Liebau MC, Lang D, Bohm J, Endlich N, Bek MJ, Witherden I, Mathieson PW, Saleem MA, Pavenstadt H, Fischer KG (2006) Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol 290:F710–F719
Bader M, Ganten D (2008) Update on tissue renin-angiotensin systems. J Mol Med 86:615–621
Takeda T (2003) Podocyte cytoskeleton is connected to the integral membrane protein podocalyxin through Na+ /H+ -exchanger regulatory factor 2 and ezrin. Clin Exp Nephrol 7:260–269
Takeda T, McQuistan T, Orlando RA, Farquhar MG (2001) Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108:289–301
Koss M, Pfeiffer GR, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM, Wang Q (2006) Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol 176:1218–1227
Schmieder S, Nagai M, Orlando RA, Takeda T, Farquhar MG (2004) Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J Am Soc Nephrol 15:2289–2298
Sagara Y, Hirooka Y, Nozoe M, Ito K, Kimura Y, Sunagawa K (2007) Pressor response induced by central angiotensin II is mediated by activation of Rho/Rho-kinase pathway via AT1 receptors. J Hypertens 25:399–406
Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S (1998) Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140:647–657
Lan M, Kojima T, Murata M, Osanai M, Takano K, Chiba H, Sawada N (2006) Phosphorylation of ezrin enhances microvillus length via a p38 MAP-kinase pathway in an immortalized mouse hepatic cell line. Exp Cell Res 312:111–120
Cant SH, Pitcher JA (2005) G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell 16:3088–3099
Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, Arpin M, Gschmeissner S, Verveer PJ, Bastiaens PI, Parker PJ (2001) Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J 20:2723–2741
Schwarz A (2001) New aspects of the treatment of nephrotic syndrome. J Am Soc Nephrol 12 Suppl 17:S44–S47
Benovic JL, Stone WC, Caron MG, Lefkowitz RJ (1989) Inhibition of the beta-adrenergic receptor kinase by polyanions. J Biol Chem 264:6707–6710
Kos CH, Le TC, Sinha S, Henderson JM, Kim SH, Sugimoto H, Kalluri R, Gerszten RE, Pollak MR (2003) Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest 111:1683–1690
Mehta PK, Griendling KK (2007) Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292:C82–C97
Hoffmann S, Krause T, van Geel PP, Willenbrock R, Pagel I, Pinto YM, Buikema H, van Gilst WH, Lindschau C, Paul M, Inagami T, Ganten D, Urata H (2001) Overexpression of the human angiotensin II type 1 receptor in the rat heart augments load induced cardiac hypertrophy. J Mol Med 79:601–608
Rossy J, Gutjahr MC, Blaser N, Schlicht D, Niggli V (2007) Ezrin/moesin in motile Walker 256 carcinosarcoma cells: signal-dependent relocalization and role in migration. Exp Cell Res 313:1106–1120
Cotton M, Boulay PL, Houndolo T, Vitale N, Pitcher JA, Claing A (2007) Endogenous ARF6 interacts with Rac1 upon angiotensin II stimulation to regulate membrane ruffling and cell migration. Mol Biol Cell 18:501–511
Acknowledgements
This work was partly funded by Chang Gung medical research project grant (CMRPG33082). Furthermore, Dr. H. H. Hsu is a recipient of the Interdepartmental Graduate-Program for Experimental Life Sciences (iGEL) fellowship from the University of Muenster, Germany, while Dr. A. Schwab was supported by a grant from IZKF Münster (Schw2/030/08) and Pa1/011/08.
This work was partially presented at the 6th International Podocyte Congress; June 8 through 11, 2006, Helsinki, Finland; the German Congress for Nephrology; September 23 through 26, 2006, Essen, Germany; and the German Congress for Nephrology; September 22 through 25, 2007, Munich, Germany.
The authors wish to thank Dr. G. Ciarimboli and U. Neugebauer (Department of Medicine D, Experimental Nephrology, University Hospital Muenster, Germany) for assisting with the intracellular calcium measurements, and S. Mally (Institute of Physiology II, University of Muenster, Germany) for assistance with life cell imaging, and Dr. C. Reinhardt (Center for Cancer Research, Department of Biology, Massachusetts Institute of Technology, MA, USA) for helping with the retroviral gene delivery method. Ted Knoy is appreciated for his editorial assistance.
Disclosures
We declare that no financial conflict of interest exists in relation to the publication of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Information 1
(PPT 612 KB)
Supplementary Information 2
(DOC 24.5 KB)
Rights and permissions
About this article
Cite this article
Hsu, HH., Hoffmann, S., Endlich, N. et al. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med 86, 1379–1394 (2008). https://doi.org/10.1007/s00109-008-0399-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-008-0399-y