Summary
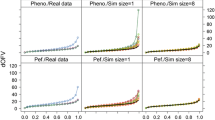
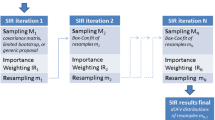
A simulation study was performed to determine how inestimable standard errors could be obtained when population pharmacokinetic analysis is performed with the NONMEM software on data from small sample size phase I studies. Plausible sets of concentration — time data for nineteen subjects were simulated using an incomplete longitudinal population pharmacokinetic study design, and parameters of a drug in development that exhibits two compartment linear pharmacokinetics with single dose first order input. They were analyzed with the NONMEM program. Standard errors for model parameters were computed from the simulated parameter values to serve as true standard errors of estimates. The nonparametric bootstrap approach was used to generate replicate data sets from the simulated data and analyzed with NONMEM. Because of the sensitivity of the bootstrap to extreme values, winsorization was applied to parameter estimates. Winsorized mean parameters and their standard errors were computed and compared with their true values as well as the non-winsorized estimates. Percent bias was used to judge the performance of the bootstrap approach (with or without winsorization) in estimating inestimable standard errors of population pharmacokinetic parameters. Winsorized standard error estimates were generally more accurate than non-winsorized estimates because the distribution of most parameter estimates were skewed, sometimes with heavy tails. Using the bootstrap approach combined with winsorization, inestimable robust standard errors can be obtained for NONMEM estimated population pharmacokinetic parameters with ≥150 bootstrap replicates. This approach was also applied to a real data set and a similar outcome was obtained. This investigation provides a structural framework for estimating inestimable standard errors when NONMEM is used for population pharmacokinetic modeling involving small sample sizes.
Similar content being viewed by others
References
Mood AM, Graybill FA, Boes DC. Introduction to the Theory of Statistics. 3rd edn, New York: McGraw-Hill, New York, 1974.
Sheiner LB, Rosenberg B, Marathe VV. Estimation of the population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm 1977; 5: 445–479 (1977)
Davidian M, Giltinan, DM. Nonlinear Models for Repeated Measurement Data. London: Chapman & Hall, 1995.
Lindstrom MJ, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics 1990; 46: 673–687.
Vonesh EF, Carter RL. Mixed-effects nonlinear regression for unbalanced repeated measures. Biometrics 1992; 48: 1–17.
Schoemaker RC, Cohen AF. Estimating impossible curves with NONMEM. Br J Clin Pharmacol 1996; 42: 283–290.
Efron B. Bootstrap methods: another look at the jackknife. Ann Statist 1979; 7: 1–26.
Efron B, Tibshirani R: Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1986; 1: 54–77.
Efron B: How biased is the apparent error rate of a prediction rule? J Am Stat Assoc 1986; 81: 461–470.
Ette EI. Population model stability and performance. J Clin Pharmacol 1997; 37: 486–495.
Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall, 1993.
Ette EI. Comparing non-hierarchical models: application to nonlinear mixed effects modeling. Computers Biol Med 1996; 26: 505–512.
Jones CD, Sun H, Ette EI. Designing cross-sectional pharmacokinetic studies: implications for pediatric and animal studies. Clin Res Regul Affairs 1996; 13 (3&4): 133–165.
Zintzaras E, Bouka P, Kowald A. Biometrical evaluation of bioequivalence trials using a bootstrap individual direct curve comparison method. Eur J Drug Metab Pharmacokinet 2002; 27: 11–16.
Bickel P, Freedman DA. Some asymptotic theory for the bootstrap. Ann Stat 1981; 9: 1196–1217.
Bickel P, Freedman DA. Asymptotic normality and the bootstrap in stratified sampling. Ann Stat 1984; 12: 470–482.
Fadiran EO, Jones CD, Ette EI. Designing population pharmacokinetic studies: performance of mixed designs. Eur J Drug Metab Pharmacokinet 2000; 25: 231–239.
Beal Sl, Sheiner LB (eds). NONMEM Users Guides, NONMEM Project Group, San Francisco, CA, 1992.
Wilcox RR. Introduction to Robust Estimation and Hypothesis Testing. New York: Academic Press, 1997.
Bickel PJ, Lehmann EL. Descriptive statistics for nonparametric models II. Location. Ann Stat 1975; 3: 1045–1069.
Ette EI, Ludden TM. Population pharmacokinetic modeling: the importance of informative graphics. Pharm Res 1995; 12 (12): 1845–1855.
Ette EI, Williams P, Sun H, et al. The Process of knowledge discovery from large pharmacokinetic data sets. J Clin Pharmacol 2001 (1); 41: 25–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ette, E.I., Onyiah, L.C. Estimating inestimable standard errors in population pharmacokinetic studies: The bootstrap with winsorization. Eur. J. Drug Metab. Pharmacokinet. 27, 213–224 (2002). https://doi.org/10.1007/BF03190460
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190460