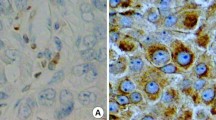
Abstract
This study describes the incidence of Bax protein expression in a series of 106 cases of breast cancer including 56 cases of ductal carcinoma in situ (DCIS) and 50 cases of invasive ductal carcinoma (IDC). Relationships of Bax expression to the histological grades of DCIS & IDC, and to the expression of Ki67, ER, p53, cerbB2 & Bcl2 are described. The expression of Bax, Ki67, ER, p53, cerbB2 and Bcl2 proteins is determined immunohistochemically. Cases were regarded positive for Bax, Bcl2 and cerbB2 when they showed either moderate or strong staining for these markers. The nuclear stains (Ki67, ER, and p53) were quantified in terms of percentage positive cells and cases for ER and p53 were considered positive when more than 10% cells were labelled. DCIS were graded histologically as well (n=18), intermediately (n=18), and poorly differentiated (n=20) Invasive ductal carcinoma was graded as grade I (well-differentiated) n=7, grade II (intermediate) n=24 and grade III (poorly differentiated) n=19. 65/106 cases (61%) were Bax positive including 37/56 (66%) of DCIS and 28/50 (56%) of IDC. Bax expression did not correlate to increasing histological grades of either DCIS or IDC. It did not correlate to Ki67, ER, p53 or cerbB2 but positive correlation was seen with Bcl2 (p= 0.003). Bcl2 immunostaining displayed a negative correlation with increasing histological grades both of DCIS and IDC (p=0.026), (p=0.041) respectively. There was a trend of negative correlation of Bcl2 with Ki67 (p=0.062). It correlated positively with Bax (p=0.003) and ER (p<0.0001). Results suggest that the regulation of apoptosis is important in ductal carcinoma in situ of the breast as well as invasive ductal carcinomas. Bcl2 is associated with good prognostic markers in both DCIS and IDC, whereas the regulation of Bax is complex and does not necessarily correlate with mutant p53.
Similar content being viewed by others
References
Oltvai ZN, Milliman CL, Korsmeyer SJ:Bcl2 heterodinerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Structure-function analysis of Blc2 protein. Cell 74:609–619, 1993.
Hanada M, Aime-Sempe C, Sato T, et al: Identification of conserved domains important for homodiverization with Bcl2 and heterodiverization with Bax. J Biol Chem 270:11962–11969, 1995.
Bargou RC, Daniel PT, Mapara MY: Expression of the bcl-2 gene family in normal and malignant breast tissue: low bax-alpha expression in tumor cells correlates with resistance towards apoptosis, Int J Cancer 60:854–859, 1995.
Korsmeyer SJ, Shutter JR, Veis DJ, et al: Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol 46:327–332, 1993.
Miyashita T, Reed JC: Tumour suppressor p53 is a direct transcriptonal activator of the human Bax gene. Cell 80:293–299, 1995.
Kapranos N, Karaiosifidi H, Valavanis C, et al: Prognostic significance of apoptosis relate1.2d proteins Bcl-2 and Bax in node-negative breast cancer patients, Anticancer Research 17:2499–505, 1997.
Tsujimoto Y, Cossman J, Jaffe E, et al: Involvement of the Bcl2 gene in human folicular lymphoma. Science 228:1440–1443, 1985.
Hockenberg DM, Zutter M, Hickey W, et al: Bcl2 protein is topgraphically restricted in tissues characterised by apoptotic cell death. Proc Natl Acad Sci 88:6961–6965, 1991.
Nathan B, Anbazhagan R, Dyer M, et al: Expression of Bcl2 like immunoreactivity in the normal breast and in breast cancer. The Breast 2:134–137, 1993.
Doglioni C, DeiTos AP, Laurino L, et al: The prevalence of Bcl2 immunoreactivity in breast carcinomas and its clinicopatholigical correlates, with particular reference to oestrogen receptor status. Virchous Arch 424:47–51, 1994.
Gee JM, Robertson JF, Ellis IO:Immunocytochemical localization of Bcl2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer 59:619–628, 1994
Silvestrini R, Veneroni S, Daidone MG: The Bcl2 protein: a prognostic indicator strongly related to p53 protein in lymphnode negative breast cancer patients. J Natl Cancer Inst 86:499–504, 1994.
Lipponen P, Pietilainen T, Kosma VM, et al: Apoptosis suppressing protein Bcl2 is expressed in well differentiated breast carcinomas with favourable prognosis. J Pathol 177:49–55, 1995.
Lane DP: p53, guardian of the genome. Nature 358:15–16, 1992.
Levine AL, Momand J, Finlay CA: The p53 tumour suppression gene. Nature 351:453–456, 1991.
Hollestein M, Sidransky D, Vogelstein B, et al: p53 mutations in human cancer. Science 253: 49–53, 1991
Haider S, Negrini M, Moune M, et al: Down regulation of Bcl2 by p53 in breast cancer cells. Cancer Res. 54:2095–2097, 1994
Kaklamanis L, Savage A, Mortensen N, et al: Early expression of Bcl2 protein in the adenoma-carcinoma sequence of colorectal neoplasia. J Pathol 179:10–14, 1996.
Shiina H, Igawa M, Urakami S, et al: Immunohistochemical analysis of Bcl2 expression in transitional cell carcinoma of the bladder. J Clin Palliol. 49:395–399, 1996
Thor AD, Moore DH II,Edgerton SM, et al: Accumulation of p53 tumour suppressor gene protein: An independent marker of prognosis in breast cancers. J. Natl Cancer Inst 84:845–855, 1992.
Coussens L, Yang-Feng TL, Liao YC, et al: Tyrosine kinase receptor with exclusive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230:1132–1139, 1985.
Wolber RA, Dupuis, BA, Wick MR: Expression of cerbB2 oncoprotein in mammary and extramammary Pagets’ disease. Am J Clin Pathol 96:243–247, 1991.
Yamamoto T, Ikawa S, Akiyama T, et al: Similarity of protein encoded by the human cerbB2 gene to epidermal growth factor receptor. Nature 319:230–234, 1986.
Allred DC, O’Connell P, Fugua AW: Biomarkers in early breast neoplasia. J. Cell Biochem 17:125–131, 1993.
Barnes DM: cerbB2 amplification in mammary carcinoma. J Cell Biochem 17:132–138, 1993.
Perren TJ: cErbB2 oncogene as a prognostic marker in breast cancer. Br J. Cancer 63:328–332, 1991.
Thompson AM:Lemoine N, Neoptolemos J, Cooke T, eds. Cancer, a molecular approach. Breast Cancer. Oxford: Blackwells 163–183, 1994
Slamon DJ, Clark GM, Wong SG, et al: Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu encogene. Science 235:177–182, 1987.
Gerdes J, Schwab U, Lemke H, et al: Production of a mouse monclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20, 1983
Nicholson RL, McClelland RA, Finlay P, et al: Relationship between EGFR, cerbB2 protein expression and ki67 immunostaining in breast cancer and hormone sensitivity. Eur J Cancer 29A:1018–1023, 1993
BouzubarN, Walker KJ, Griffiths K, et al: Ki67 immunostaining in primary breast cancer: pathological and clinical associations. Br J Cancer 59:943–947, 1989.
Pinder SE, Wencyk P, Sibbering DM, et al: Assessment of the new proliferation marker MIBI in breast carcinoma using image analysis; associations with other prognostic factors and survival. Br J Cancer 71:146–149, 1995.
Locker AP, Birrell K, Bell JA, et al: Ki67 immunoreactivity in breast carcinoma: relationship to prognostic variables and short term survival. Eur J Surg Oncol 1992; 18:224–229, 1992.
Nicholson RI, Bouzubar N, Walker KJ, et al: Hormone sensitivity in breast cancer: influence of heterogeneity of oestrogen receptor expression and cell proliferation. Eur J Cancer 27:908–913, 1991
Thorpe SM, Rose C, Rasmussen BB, et al: Prognostic value of steroid hormone receptor multivariate analysis of systemically untreated patients with node negative primary breast cancer. Cancer Res 47:6126–6133, 1987.
Kiang DT, Frenning DH, Goldman AL, et al: Estrogen receptors and responses to chemotherapy and hormonal therapy in advanced breast cancer. N Engl J Med 299:1330–1333, 1978.
Anderson J, Thorp SM, King WJ, et al:The prognostic value of immunohistochemical oestrogen receptor analysis in paraffin embedded and frozen sections versus that of steroid binding assays. Eur J Cancer 26:442–449, 1990.
Elledge RM, Green S, Howes L, et al: Bcl2, p53 and response to lamonifen in oestrogen receptor positive metastatic breast cancer: a southwest oncology group study. J Clin Oncol 15:1916–1922, 1997.
Holland R, Peterse JL, Millis RR, et al: Ductal carcinoma in situ: A proposal for a new classification. Seminars in Diagnostic Pathology. 11:167–180, 1994.
Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long term follow up. Histopathology; 19:403–410, 1991
Vilain MO, Delobelle-Deroide A, Bloget F, et al: Immunohistochemical detection of oestrogen and progesterone receptors in formation fixed, paraffin embedded tissues after microwave treatment. Comparison with biochemical assay in a series of 123 breast carcinomas with determination of the positivity cut off. Annales de Pathologie. 17:82–88, 1997.
Krajewski S, Krajewska M, Shabaik A, et al: Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl2. Am J Pathol; 145:1323–1336, 1994.
World Health Organisation, (1981) Histological typing of breast tumours. International Histological Classification of Tumours, 2nd Edition World Health Organisation, Geneva.
Reed JC: Bcl2 and the regulation of programmed cell death. J Cell Biol 124:1–6, 1994.
Hockenberg DM: Bcl2 in cancer, development and apoptosis. J Cell Sci 18:51–55, 1994.
Kerr JF, Winterford CM, Harmon BV: Apoptosis — its significance in cancer and cancer therapy. Cancer 73:2013–2026, 1994.
White E: Life, death and the pursuit of apoptosis. A review. Genes and Development 10:1–15, 1996.
Yang E, Korsmeyer SK: Molecular thanatopsis: a discourse on the Bcl2 family and cell death. Blood 88:386–401, 1996.
Thompson CB: Apoptosis in the pathogensis and the treatment of disease. Science 267:1456–1462, 1995.
Broise LH, Gottschalk AR, Quinfans J, Thompson CB:Bcl2 and Bcl2 related proteins in apoptosis regulation. Curr Top Microbiol Immunol 200:107–121, 1995.
Craig RW: The Bcl2 gene family. Semin Cancer Biol 6:35–43, 1995.
Krajewski S, Blomqvist C, Franssila K, et al: Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res 55:4471–4478, 1995
Kapucuoglu N, Losi L, Eusebi V: Immunohistochemical localization of Bcl-2 and Bax proteins in in situ and invasive duct breast carcinomas. Virchows Arch 430:17–22, 1997
Yang Q, Sakurai T, Jing X, et al: Expression of Bcl2, but not Bax, correlates with estrogen receptor status and tumour proliferation in invasive breast carcinoma, Pathology International, 49;775–780, 1999.
Rochaix P, Krajewski S, Reed JC, et al: In vivo patterns of Bcl2 family protein expression in breast carcinoma in relation to apoptosis. J Pathol 87:410–415, 1999.
Van Slooten HJ, Clahsen PC, Van Dierendonck JH: Expression of Bcl2 in node negative breast cancer is associated with various prognostic factors, but does not predict response to one course of peri-operative chemotherapy, Br J Cancer 74:78–85, 1996.
Joensuu H, Pylkkanen L, Loikkanen S: Bcl2 protein expression and long term survival in breast cancer, Amer J Pathol 145:1191–1198, 1994.
Siziopikou KP, Prioleau JE, Harris JR, et al: Bcl2 expression in the spectrum of pre-invasive breast lesions. Cancer 77:499–506, 1996
Quinn CM, Ostrowski JL, Harkins L, et al: Loss of Bcl2 expression in DCIS of the breast relates to poor histological differentiation and to expression of p53 and cerbB2 proteins. Histopathology 3:531–536, 1998.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehman, S., Crow, J. & Revell, P.A. Bax protein expression in DCIS of the breast in relation to invasive ductal carcinoma and other molecular markers. Pathol. Oncol. Res. 6, 256–263 (2000). https://doi.org/10.1007/BF03187328
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03187328