Summary
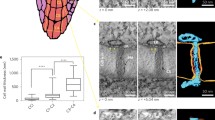
The substructure of plasmodesmata in freeze-substituted tissues of developing leaves of the tobacco plant (Nicotiana tabacum L. var. Maryland Mammoth) was studied by high resolution electron microscopy and computer image enhancement techniques. Both the desmotubule wall and the inner leaflet of the plasmodesmatal plasma membrane are composed of regularly spaced electron-dense particles approximately 3 nm in diameter, presumably proteinaceous and embedded in lipid. The central rod of the desmotubule is also particulate. In plasmodesmata with central cavities, spoke-like extensions are present between the desmotubule and the plasma membrane in the central cavity region. The space between the desmotubule and the plasma membrane appears to be the major pathway for intercellular transport through plasmodesmata. This pathway may be tortuous and its dimensions could be regulated by interactions between desmotubule and plasma membrane particles.
Similar content being viewed by others
Abbreviations
- ER:
-
endoplasmic reticulum
- PJF:
-
propane jet freezing
- HPF:
-
high pressure freezing
- CRT:
-
cathode ray tube
- IP3 :
-
inositoltrisphosphate
References
Baron-Epel O, Hernandez D, Jiang LW, Meiners S, Schindler M (1988) Dynamic continuity of cytoplasmic and membrane compartments between plant cells. J Gell Biol 106: 715–721
Burgess J (1971) Observations on structure and differentiation in plasmodesmata. Protoplasma 73: 83–95
Calvert HE, Pence MK, Peters GA (1986) Ultrastructural ontogeny of leaf cavity trichomes inAzolla implies a functional role in metabolite exchange. Protoplasma 129: 10–27
Ding B, Parthasarathy MV, Niklas K, Turgeon R (1988) A morphometric analysis of the phloem unloading pathway in developing tobacco leaves. Planta 176: 307–318
—, Turgeon R, Parthasarathy MV (1991 a) Plasmodesmatal substructure in cryofixed developing tobacco leaf tissue. In: Bonnemain JL, Delrot S, Dainty J, Lucas WJ (eds) Recent advances in phloem transport and assimilate compartmentation. Quest Editions, Nantes, pp 317–323
— — — (1991 b) Routine cryofixation of plant tissues by propane jet freezing for freeze substitution. J Electron Microsc Techn 19: 107–117
— — — (1991 c) Microfilament organization and distribution in freeze substituted tobacco plant tissues. Protoplasma 165: 96–105
Erwee MG, Goodwin PB (1983) Characterization of theEgeria densa Planch, leaf symplast. Inhibition of the intercellular movement of fluorescent probes by group II ions. Planta 158: 320–328
Evert RF, Eschrich W, Heyser W (1977) Distribution and structure of plasmodesmata in mesophyll cells ofZea mays L. Planta 136: 77–89
Fujiwara K, Link RW (1982) The use of tannic acid in microtubule research. In: Wilson L (ed) Methods in cell biology. Academic Press, New York, pp 217–233
Futaesaku Y, Mizuhira V, Nakamura H (1972) The new fixation method using tannic acid for electron microscopy and some observations of biological specimens. In: Proceedings of the IVth International Congress for Histochemistry and Cytochemistry, pp 155–166
Goodwin PB (1983) Molecular size limit for movement in the symplast of theElodea leaf. Planta 157: 124–130
Gunning BES, Hughes JE (1976) Quantitative assessment of symplastic transport of pre-nectar into the trichomes ofAbutilon nectaries. Aust J Plant Physiol 3: 619–637
—, Overall RL (1983) Plasmodesmata and cell-to-cell transport in plants. BioScience 33: 260–265
, Robards AW (eds) (1976) Intercellular communication in plants: studies on plasmodesmata. Springer, Berlin Heidelberg New York
Hepler PK (1982) Endoplasmic reticulum in the formation of the cell plate and plasmodesmata. Protoplasma 111: 121–133
Lancelle SA, Cresti M, Hepler PK (1987) Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes ofNicotiana alata. Protoplasma 140: 141–150
Lopez-Saez JF, Gimenez-Martin G, Risueno MC (1966) Fine structure of the plasmodesm. Protoplasma 61: 81–84
Markham R, Frey S, Hills GJ (1963) Methods for the enhancement of image detail and accentuation of structure in electron microscopy. Virology 20: 88–102
Millonig G, Marinozzi V (1968) Fixation and embedding in electron microscopy. In: Barer R, Cosslett VE (eds) Advances in optical and electron microscopy, vol 2. Academic Press, New York, pp 251–341
Mollenhauer HH, Morré DJ (1987) Some unusual staining properties of tannic acid in plants. Histochemistry 88: 17–22
Olesen P (1979) The neck constriction in plasmodesmata evidence for a peripheral sphincter-like structure revealed by fixation with tannic acid. Planta 144: 349–358
—, Robards AW (1990) The neck region of plasmodesmata: general architecture and some functional aspects. In: Robards AW, Jongsma H, Lucas WJ, Pitts J, Spray D (eds) Parallels in cell to cell junctions in plants and animals. Springer, Berlin Heidelberg New York Tokyo, pp 145–170
Overall RL, Wolfe J, Gunning BES (1982) Intercellular communication inAzolla roots: I. Ultrastructure of plasmodesmata. Protoplasma 111: 134–150
Paine PL, Moor LC, Horowitz SB (1975) Nuclear envelope permeability. Nature 254: 109–114
Palevitz BA, Hepler PK (1985) Changes in dye coupling of stomatal cells ofAllium andCommelina demonstrated by microinjection of Lucifer Yellow. Planta 164: 473–479
Robards AW (1968) A new interpretation of plasmodesmatal ultrastructure. Planta 82: 200–210
— (1971) The ultrastructure of plasmodesmata. Protoplasma 72: 315–323
— (1976) Plasmodesmata in higher plants. In: Gunning BES, Robards AW (eds) Intercellular communication in plants: studies on plasmodesmata. Springer, Berlin Heidelberg New York, pp 15–57
—, Lucas WJ (1990) Plasmodesmata. Annu Rev Plant Physiol Plant Mol Biol 41: 369–419
Scalet M, Crivellato E, Mallardi F (1989) Demonstration of phenolic compounds in plant tissues by an osmium-iodide postfixation procedure. Stain Technol 64: 273–280
Seagull RW, Heath IB (1979) The effect of tannic acid on the in vivo preservation of microfilaments. Eur J Cell Biol 20: 184–188
— — (1980) The differential effects of cytochalasin B on microfilament populations and cytoplasmic streaming. Protoplasma 103: 231–240
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Terry BR, Robards AW (1987) Hydrodynamic radius alone governs the mobility of molecules through plasmodesmata. Planta 171: 145–157
Thomson WW, Platt-Aloia WW (1985) The ultrastructure of the plasmodesmata of the salt glands ofTamarix aphylla as revealed by transmission electron microscopy and freeze fracture electron microscopy. Protoplasma 125: 13–23
Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Snyder DH (1973) Microtubules: evidence for 13 protofilaments. J Cell Biol 59: 267–275
—, Cooke TJ, Connelly PS, Tilney MS (1991) The structure of plasmodesmata as revealed by plasmolysis, detergent extraction, and protease digestion. J Cell Biol 122: 73–747
Tiwari SC, Polito VS (1988) Organization of the cytoskeleton in pollen tubes ofPyrus communis: a study employing conventional and freeze-substitution electron microscopy, immunofluorescence, and rhodamine-phallaidin. Protoplasma 147: 100–112
—, Wick SM, Williamson RE, Gunning BES (1984) Cytoskeleton and integration of cellular function in cells of higher plants. J Cell Biol 99: 63s-69s
Tucker EB (1988) Inositol bisphosphate and inositol triphosphate inhibit cell-to-cell passage of carboxyfluorescein in staminal hairs ofSetcreasea purpurea. Planta 174: 358–363
— (1990) Calcium-loaded 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid blocks cell-to-cell diffusion of carboxyfluorescein in staminal hairs ofSetcreasea purpurea. Planta 82: 34–38
Turgeon R (1984) Termination of nutrient import and development of vein loading capacity of albino tobacco leaves. Plant Physiol 76: 45–48
— (1987) Phloem unloading in tobacco sink leaves: insensitivity to anoxia indicates a symplastic pathway. Planta 171: 73–81
Willison JHM (1976) Plasmodesmata: a freeze fracture view. Can J Bot 54: 2842–2847
Yahalom A, Warmbrodt RD, Laird DW, Traub O, Revel JP, Willecke K, Epel BL (1991) Maize mesocotyl plasmodesmata proteins crossreact with connexin gap junction protein antibodies. Plant Cell 3: 407–417
Zee SY (1969) The fine structure of differentiating sieve elements ofVicia faba. Aust J Bot 17: 441–456
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ding, B., Turgeon, R. & Parthasarathy, M.V. Substructure of freeze-substituted plasmodesmata. Protoplasma 169, 28–41 (1992). https://doi.org/10.1007/BF01343367
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01343367