Summary
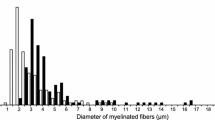
The relative changes in the growth and maturation of axon size and myelin thickness were studied in the medial plantar division of the tibial nerve in the lower leg and in the motor branches of the tibial nerve to the calf muscles in rats in which diabetes mellitus had been induced with streptozotocin at the time of weaning. Observations were made at 6 weeks and 3, 6, 9 and 12 months of diabetes for comparison with age-matched controls. Similar changes were observed in both nerves. Growth in body weight and skeletal growth was severely retarded from the time of induction of diabetes but at the 6-week stage axon size was not reduced, suggesting that neural growth may initially be relatively protected. At later stages axon size was consistently reduced in the diabetic animals as compared with the controls and showed an absolute reduction at 12 months, as compared with 9 months, that was greater than in the controls. Myelin thickness became reduced earlier and was more severely affected than axon size so that the fibers were relatively hypomyelinated. The myelin changes were greater in larger than in smaller fibers. The index of circularity of axons was reduced in the diabetic nerves. These results show that induction of diabetes in prepubertal rats produces effects on peripheral nerve fibers which differ from those resulting from diabetes induced in adult animals. The effects also differ between large and small nerve fibres. These observations may explain some of the disparate findings obstained in previous studies on experimental diabetes in rats.
Similar content being viewed by others
References
Bestetti C, Zemp C, Probst D, Rossi GL (1981) Neuropathy and myopathy of rats after 12 months of streptozotocin-induced diabetes mellitus. A light, electron microscopic and morphometric study. Acta Neuropathol (Berl) 55: 11–20
Beuche W, Friede RL (1985) A new approach toward analyzing peripheral nerve fiber populations. II. Foreshortening of regenerated internodes corresponds to reduced sheath thickness. J Neuropathol Exp Neurol 44: 73–84
Beuche W, Friede RL (1986) Remodelling of nerve structure in experimental isoniazid neuropathy in the rat. Brain 109: 759–769
Bhoyrul S, Sharma AK, Stribling D, Mirrlees DD, Peterson RG, Farber MO, Thomas PK (1988) Ultrastructural observations on myelinated fibres in experimental diabetes: effect of the aldose reductase inhibitor ponalrestat given alone or on conjunction with insulin therapy. J Neurol Sci 85: 131–148
Birren JE, Wall PD (1956) Age changes in conduction velocity, refractory period, number of fibers, connective tissue space and blood vessels of sciatic nerve of rats. J Comp Neurol 104: 1–16
Cameron NE, Leonard MB, Ross IS, Whiting PH (1986) The effect of sorbinil on peripheral nerve conduction velocity, polyol concentrations and morphology in streptozotocin-diabetic rats. Diabetologia 29: 168–174
Chokraverty S, Reyes MG, Seiden D, Nishimura N (1980) Axonal neuropathy in acute streptozotocin diabetes. Ann Neurol 8: 104–105
Dyck PJ, Lambert EH, Windebank AJ, Lais AA, Sparks MT, Karnes J, Sherman WR, Hallcher LH, Low PA, Service FJ (1980) Acute hyperosmolar hyperglycemia causes axonal shrinkage and reduced nerve conduction velocity. Exp Neurol 71: 507–514
Fraher JP (1989) Axon-myelin relationships in rat cranial nerves III, IV and VI. A morphometric study of large and small fibre classes. J Comp Neurol (in press)
Fraher JP, Kaar GF (1985) The development of alpha and gamma motoneuron fibres in the rat. II. A comparative ultrastructural study of their central and peripheral myelination. J Anat 141:89–103
Fraher JP, O'Leary D, Moran MA, Cole M, King RHM, Thomas PK (1990) Relative growth and maturation of axon size and myelin thickness in the tibial nerve of the rat. 1. Normal animals. Acta Neuropathol 79: 364–374
Friede RL, Beuche W (1985) Combined scatter diagrams of sheath thickness and fibre calibre in human sural nerves: changes with age and neuropathy. J Neurol Neurosurg Psychiatry 48: 749–756
Friede RL, Bischhausen R (1982) How are sheath dimensions affected by axon calibre and internode length? Brain Res 235: 335–350
Friede RL, Brzoska J, Hartmann U (1985) Changes in myelin sheath thickness and internode geometry in the rabbit phrenic nerve during growth. J Anat 143: 103–113
Friede RL, Meier T, Diem M (1981) How is the exact length of an internode determined? J Neurol Sci 50: 217–228
Gabbay KH (1973) The sorbitol pathway and complications of diabetes. N Engl J Med 288: 831–836
Hanrankova J, Roth J, Brownstein M (1978) Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272: 827–829
Hill JM, Lesniak MA, Pert CB, Roth J (1986) Autoradiographic localization of insulin receptors in rat brain: prominence in limbic and olfactory areas. Neuroscience 17: 1127–1138
Hoffman PN, Griffin JW, Price DL (1984) Control of axonal caliber by neurofilament transport. J Cell Biol 99: 705–714
Jacobs JM, Love S (1985) Qualitative and quantitative morphology of the human sural nerve at different ages. Brain 108: 897–924
Jakobsen J (1976) Axonal dwindling in early experimental diabetes. I. A study of cross-sectioned nerves. Diabetologia 12: 539–546
Jakobsen J (1976) Axonal dwindling in early experimental diabetes. II. A study of isolated nerve fibres. Diabetologia 12: 547–553
Jakobsen J, Sidenius P (1980) Transport of structural proteins in streptozotocin diabetic rats. J Clin Invest 66: 292–297
Kappy M, Sellinger S, Raizada M (1984) Insulin binding in four regions of the rat brain. J Neurochem 42: 192–203
King RHM, Craggs RI, Gross MLP, Tompkins C, Thomas PK (1983) Suppression of experimental allergic neuritis by cyclosporin A. Acta Neuropathol (Berl) 59: 262–268
Llewelyn JG, Patel NJ, Thomas PK, Thompson CS, Muddle JR, Workman JM, Dashwood MR (1988) Insulin receptors in peripheral nerve tissues. J Neurol 235: S16
Mattingly GE, Fischer VW (1985) Peripheral nerve axonal dwindling with concomitant myelin sheath hypertrophy in experimentally induced diabetes. Acta Neuropathol (Berl) 68: 149–154
McCallum KNC, Sharma AK, Blanchard DS, Stribling D, Mirrlees DJ, Duguid IGM, Thomas PK (1986) The effect of continuous subcutaneous insulin therapy on morphological and biochemical abnormalities of peripheral nerves in experimental diabetes. J Neurol Sci 74: 55–67
Medori R, Autilio-Gambetti L, Monaco S, Gambetti P (1985) Experimental diabetic neuropathy: impairment of slow transport with changes in axonal cross-sectional area. Proc Natl Acad Sci USA 82: 7716–7720
Morgane FJ, Miller M, Kempner T, Stern W, Forbes W, Hall R, Bronzino J, Kissane J, Hawrylewicz E, Resnick O (1978) The effect of protein-calorie malnutrition on the developing nervous system in the rat. Neurosci Biobehav Rev 2: 137–230
Oldfors A, Ullman M (1980) Motor nerve conduction velocity and nerve fibre diameter in experimental protein deprivation. Studies on rat peripheral nerve during development. Acta Neuropathol (Berl) 51: 215–221
Phillips LS, Young HS (1976) Nutrition and somatomedin. II. Serum somatomedin activity and cartilage growth activity in streptozotocin diabetic rats. Diabetes 25: 516–527
Phillips LS, Bajaj VR, Fuseo AC, Matheson CK (1983) Nutrition and somatomedin. XI. Studies on somatomedin inhibitors in rats with streptozotocin-induced diabetes. Diabetes 32: 1117–1125
Sharma AK, Thomas PK (1974) Peripheral nerve structure and function in experimental diabetes. J Neurol Sci 23: 1–15
Sharma AK, Bajada S, Thomas PK (1981) Influence of streptozotocin-induced diabetes on myelinated nerve fibre maturation and on body growth in the rat. Acta Neuropathol (Berl) 53: 257–265
Sidenius P, Jakobsen J (1982) Reversibility and preventability of the decrease in slow axonal transport velocity in experimental diabetes. Diabetes 31: 689–693
Sima AAF, Zhang W-X, Tze WJ, Tai J, Nathaniel V (1988) Diabetic neuropathy in STZ-induced diabetic rat and effect of allogeneic islet cell transplantation: morphometric analysis. Diabetes 37: 1129–1136
Sugimura K, Windebank AJ, Natarajan V, Lambert EH, Schmid HO, Dyck PJ (1980) Interstitial hyperosmolarity may cause axis cylinder shrinkage in streptozotocin diabetic nerve. J Neuropathol Exp Neurol 39: 710–721
Taylor AM, Sharma AK, Avasthy N, Duguid IGM, Blanchard DS, Thomas PK, Dandona P (1987) Somatomedin inhibition by serum from streptozotocin-diabetic rats: preventation by insulin treatment and correlation with skeletal growth. Endocrinology 121: 1360–1365
Tomlinson DR, Sidenius P, Larsen JR (1986) Slow component-a of axonal transport, nerve myo-inositol, and aldose reductase inhibition in streptozotocin-diabetic rats. Diabetologia 35: 398–402
Waldbillig RJ, LeRoith D (1987) Insulin receptors in the peripheral nervous system: a structural and functional analysis. Brain Res 409: 215–220
Winegrad AI, Simmons DA (1987) Energy metabolism in peripheral nerve. In: Dyck PJ, Thomas PK, Asbury AK, Winegrad AI, Porte J (eds) Diabetic neuropathy. WB Saunders, Philadelphia, pp 279–288
Zemp C, Bestetti G, Rossi GL (1981) Morphological and morphometric study of peripheral nerves from rats with streptozotocin-induced diabetes mellitus. Acta Neuropathol (Berl) 53: 99–106
Author information
Authors and Affiliations
Additional information
Supported by an EEC Twinning Grant and from the Nuffield Foundation
Rights and permissions
About this article
Cite this article
Thomas, P.K., Fraher, J.P., O'Leary, D. et al. Relative growth and maturation of axin size and myelin thickness in the tibial nerve of the rat. Acta Neuropathol 79, 375–386 (1990). https://doi.org/10.1007/BF00308713
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00308713