Summary
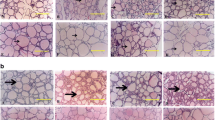
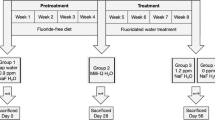
The parathyroid glands of young male rats given 150 ppm fluoride in their drinking water for 10 weeks were examined by transmission electron microscopy. As a result of fluoride ingestion, the parathyroid chief cells of the experimental animals accumulated glycogen in excess of that seen in control animals given distilled drinking water for the same time period. In the majority of active chief cells, glycogen granules were diffusely spread throughout the cytoplasm as single granules or in small deposits. Large aggregations of glycogen granules were also seen within intercellular spaces. Accompanying the increase in glycogen was a rise in the number and development of the organelles associated with protein synthesis and secretion. The accumulation of glycogen is similar to that in hyperparathyroidism caused by chronic stimulation and prolonged secretory activity of the parathyroid gland. The results of this study suggest that increased amounts of glycogen occur in hyperactive chief cells of the parathyroid in response to the ingestion of large doses of fluoride.
Similar content being viewed by others
References
Altenahr E (1972) Ultrastructural pathology of parathyroid glands. Curr Top Pathol 56:1–54
Baylink DJ, Bernstein DS (1967) The effects of fluoride therapy on metabolic bone disease. Clin Orthop 55:51–85
Bernstein DS, Cohen P (1967) Use of sodium fluoride in the treatment of osteoporosis. J Clin Endocrinol 27:197–210
Capen CC, Rowland GN (1968) Ultrastructural evaluation of the parathyroid glands of young cats with experimental hyperparathyroidism. Z Zellforsch 90:495–506
Capen CC, Koestner A, Cole CR (1965) The ultrastructure and histochemistry of normal parathyroid glands of pregnant and nonpregnant cows. Lab Invest 14:1673–1690
Elliott RL, Arhelger RB (1966) Fine structure of parathyroid adenomas. Arch Pathol 81:200–212
Faccini JM (1967) Inhibition of bone resorption in the rabbit by fluoride. Nature 214:1269–1271
Faccini JM, Care AD (1965) Effects of sodium fluoride on the ultrastructure of the parathyroid glands of the sheep. Nature 207:1399–1401
Fujimoto Y, Matsukawa K, Inubushi H, Nakamatsu M, Satoh H, Yamigiwa S (1967) Electron microscopic observations of the equine parathyroid glands with particular reference to those of equine osteodystrophia fibrosa. Jap J Vet Res 15:37–51
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Krook L, Lowe JE (1964) Nutritional secondary hyperparathyroidism in the horse with a description of the normal equine parathyroid gland. Pathol Vet 1:1–98
Lever JD (1957) Fine structural appearances in the rat parathyroid. J Anat 91:73–81
Marshall RB, Roberts DK, Turner RA (1967) Adenomas of the human parathyroid. Light and electron microscopic study following selenium 75 methionine scan. Cancer 20:512–524
Millonig G (1961) Advantages of a phosphate buffer for OsO4 solutions in fixation. J Appl Phys 32:1637
Munger BL, Roth SI (1963) The cytology of the normal parathyroid gland of man and Virginia deer. J Cell Biol 16:379–400
Nilsson O (1977) Studies on the ultrastructure of the human parathyroid glands in various pathological conditions. Acta Pathol Microbiol Scand 263:1–88
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Roth SI (1962) Pathology of the parathyroids in hyperparathyroidism. Arch Pathol 73:75–90
Roth SI, Munger BL (1962) The cytology of the adenomatous, atrophic and hyperplastic parathyroid glands of man. Virchows Arch Path Anat 335:389–410
Roth SI, Raisz LG (1964) Effect of calcium concentration on the ultrastructure of rat parathyroid in organ culture. Lab Invest 13:331–345
Roth SI, Raisz LG (1966) The course and reversibility of the calcium effect on the ultrastructure of the rat parathyroid gland in organ culture. Lab Invest 15:1187–1211
Roth SI, Capen CC (1974) Ultrastructural and functional correlation of the parathyroid gland. Int Rev Exp Pathol 13:161–221
Teotia SPS, Teotia M (1973) Secondary hyperparathyroidism in patients with endemic skeletal fluorosis. Brit Med J 1:637–640
Thiele J (1977) Human parathyroid gland: a freeze fracture and thin section study. Curr Top Pathol 65:31–80
Weymouth RJ, Sheridan MN (1966) Fine structure of human parathyroid glands: normal and pathological. Acta Endocrin 53:529–546
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ream, L.J., Principato, R. Glycogen accumulation in the parathyroid gland of the rat after fluoride ingestion. Cell Tissue Res. 220, 125–130 (1981). https://doi.org/10.1007/BF00209971
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00209971